38 lewis dot diagram for nacl
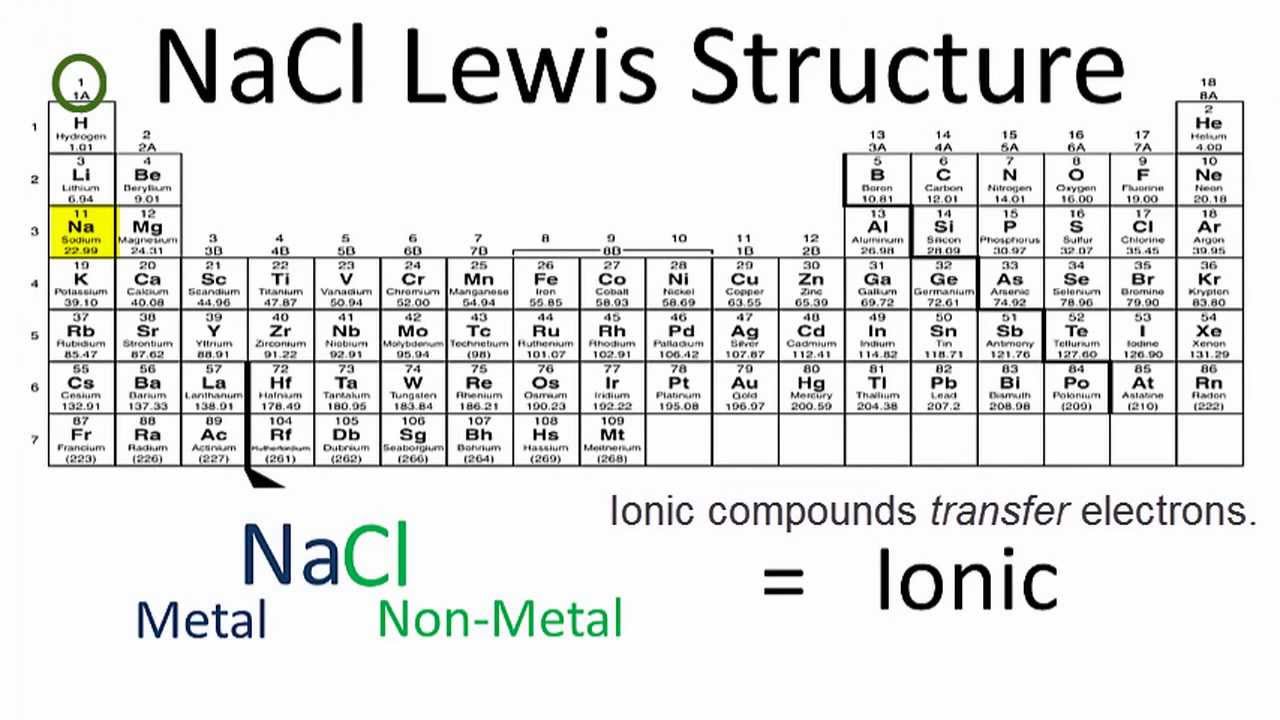
9 Covalent; difference in electronegativity is small (less than 1.67) What type of bond exists between chlorine (EN = 3.0) and bromine (EN = 2.8)? Give a ...Jun 28, 2018 The total valence electron is available for drawing the Sodium chloride (NaCl) lewis structure is 8. · NaCl is a face-centered cubic unit cell that has four ...Name of Molecule: Sodium chlorideCoordination number: 6:6Crystal structure: Face-centered cubicHow to draw lewis structure for... · Steps to draw electron dot...
The Lewis dot symbol for the chloride ion is. Lets do the Lewis structure for H3O the hydronium ion. Lets do the Lewis structure for H3O the hydronium ion. Posted on January 2 2021 by. Note that the sign in the Lewis structure for H3O means that we have lost a valence electron.

Lewis dot diagram for nacl
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc... Oct 21, 2014 — The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
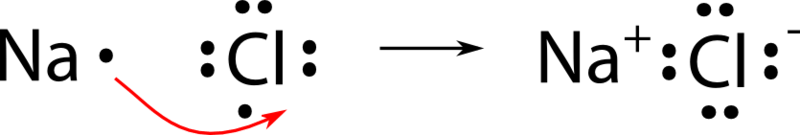
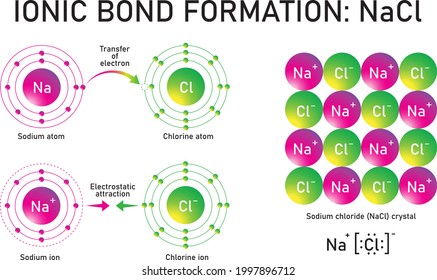
Lewis dot diagram for nacl. Sodium chloride lewis dot diagram. Diagram of bonding in sodium chloride. The hypotonic 045 sodium chloride irrigation may be used alone or with appropriate additives when 09 sodium chloride irrigation is considered too irritating for wounds or other altered structures. The lewis dot structure for francium up with chlorines unpaired dot. A. The Lewis dot structure for francium up with chlorine's unpaired dot. Chemists often depict a bond with a line, so sodium chloride can be written as Na -Cl. Draw the correct Lewis dot structure for CH2O & determine the shape Trigonal Planar. A sodium ion (2,8)+. Diagram of bonding in sodium chloride. A sodium atom gives an electron to a ... The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. The lewis dot structure of nacl consists of a chloride ion surrounded by eight electron dots four pairs and a sodium ion bonded to that chlorine ion. Ionic bonding explained what is an bond electron transfer how.
Atoms in molecules (especially carbon) are often described as being. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Transcribed Image Textfrom this Question. Write a Lewis structure for sodium chloride, NaCl, showing all valence electrons. . • You do not have to consider stereochemistry. Explicitly draw all H atoms. • Include all valence lone pairs in your answer. • Draw cations and anions in separate sketchers. • Separate structures with + signs ... In this case, it's relatively simple because sodium has only one valence electron. Remember, you don't want any unpaired dots, so sodium's dot will join up with ... Sep 3, 2019 — where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the ...
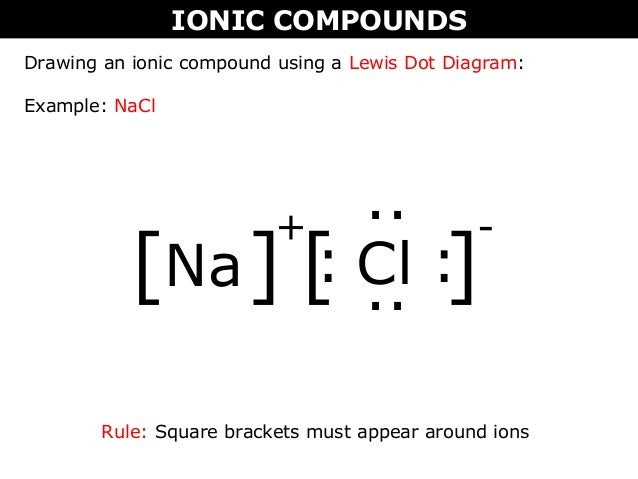
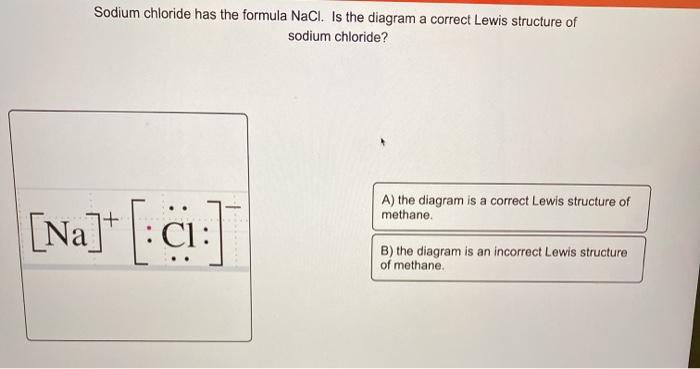
Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell! The Lewis Structure for the Salt NaCl,shows two ions which have their (Now) outer shells of electronsfilled with a complete octet.In the case of the sodium cation, thefilled shell is the outermost of the 'core' electron shells. In theChloride ion, the outer shell of valence electrons is complete with8 electrons. This structure is called the "NaCl Structure"! Several other ionic compounds have the same structure, but it is named after NaCl since that is such a common compound. What is the Lewis structure ... The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1. The Lewis dot structure of ionic bonds such as NaCl is formed by looking at both ...
Check me out: http://www.chemistnate.com
For example, consider sodium chloride. The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells.
Salt (Sodium Chloride) Lewis dot structure for NaCl. Wiki User. ∙ 2014-07-25 16:29:22. Study now. See Answer. Best Answer. Copy. I cannot transfer and save the picture here; so, see the link below.
A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". The atomic number of "Na" is 11, so it has 11 electrons.
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The lines are a short-hand version of the two dots representing the covalent bonds.
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure.For the NaCl Lewis structure, calculate the total number of valence electrons for the ...
Aug 16, 2012 — table salt, NaCl. Procedure for a Covalent Compound: 1. Draw the Lewis dot structure for each atom of the molecule to show how many valence.3 pages
1 answerThe structure of dot representation for the formation of NaCl is written above. expand. Solve any question of Chemical Bonding and Molecular Structure with ...
Lewis dot diagram for nacl. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist. Falcons school for girls sobao for ever lewis mckenzie melissa lewis see more. By snoopy for life. Cant really draw it on here 1. The lewis dot structure of nacl consists of a chloride ion ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Oct 21, 2014 — The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet.
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc...

























0 Response to "38 lewis dot diagram for nacl"
Post a Comment