38 o2 lewis dot diagram
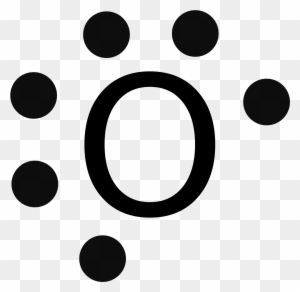
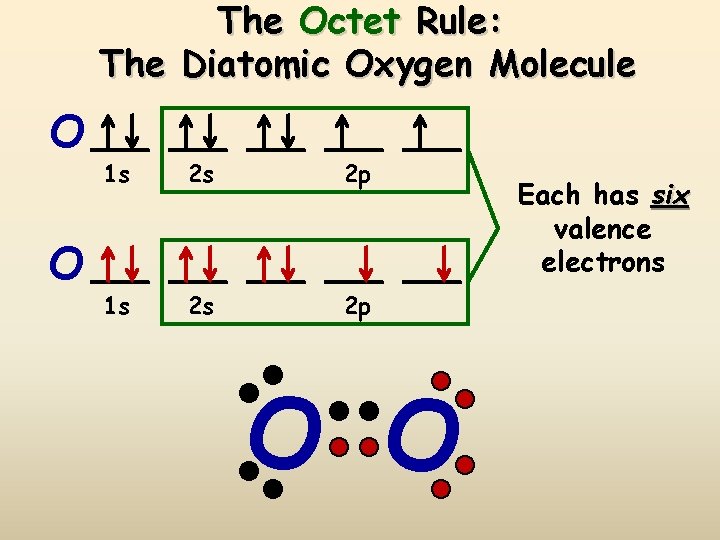
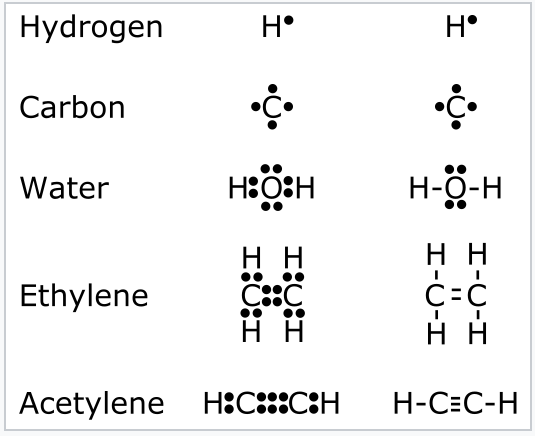
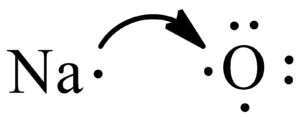
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. So as you may of remember from Chemistry ...
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
O2 lewis dot diagram
Lewis Dot Structure: The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...
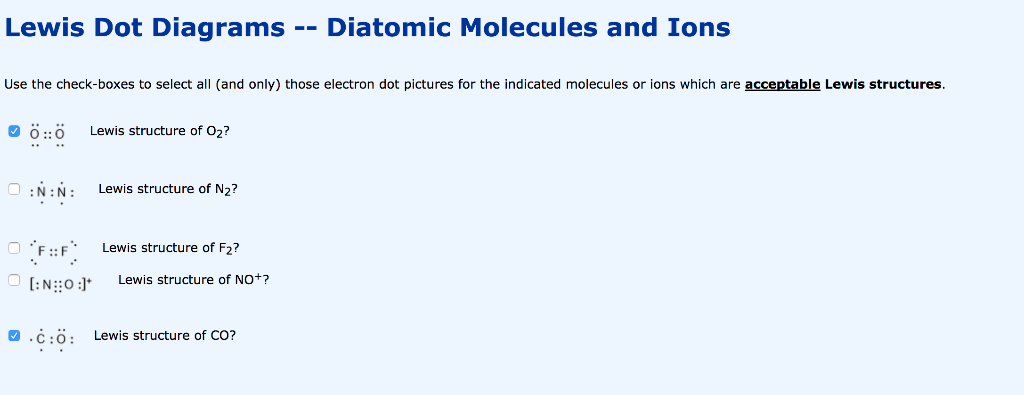
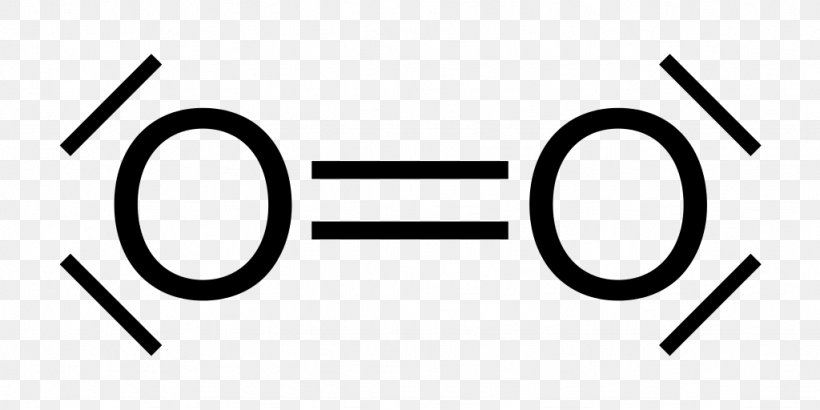
O2 lewis dot diagram. Answer (1 of 4): The easiest way to them is in steps: Step 1: Count number of total Valance electrons (12 electrons in this case) Step 2: No. of Required electrons (always 8 hence, 16 electrons) Step 3: No. of Bonding Electrons (Required electrons - valence electrons: 4 electrons in this case)... Drawing the Lewis Structure for O 2 (Dioxygen or Oxygen Gas). Oxygen (O 2) is a commonly tested Lewis structure due to it's importance on Earth.It also is a good example of a molecule with a double bond. There are 12 valence electrons available for the Lewis structure for O 2.. Video: Drawing the Lewis Structure for O 2 Lewis Structure (electron dot diagram) for the oxygen molecule, O 2, OR . There are 2 bonding pairs of electrons shared between the 2 oxygen atoms, and each oxygen atom also has 2 lone pairs (non-bonding) pairs of electrons. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
This is the Lewis Dot Structure for O2, commonly referred to as oxygen gas. Due to oxygen's high electronegativity (affinity for electrons), the pure element is nearly exclusively found in either this state or ozone (O3 - a distinct lewis structure for another post). When drawing the structure, you may replace the individual lines with two dots symbolizing the two electrons contained within ... Silicon dioxide (SiO2) lewis structure. As you see in the above SiO2 lewis dot structure, we convert 2 lone pairs of electrons of each oxygen atom to a covalent bond. So, both atoms (silicon and oxygen) have 8 electrons in their valence shell. Hence we got our best and stable Silicon dioxide lewis structure. The Lewis Dot Structure for O2 or dioxygen is as follows: O = O. ADVERTISEMENT. It’s a very simple structure, but how does one interpret this Lewis structure? How can one draw a Lewis structure and use it to understand how atoms bond together to make molecules? Let’s go over how Lewis structures are interpreted and drawn. Facts About Oxygen (O2) Photo: Benjah-bmm27 via Wikimedia Commons ... Nitrate ion lewis dot structure. In brief we need to master 4 steps for making a correct Lewis dot structure. Count total valence electrons in the molecule or ion. Select the central atom and make a skeleton of the molecule or ion. Complete the octet of the most electronegative atom with minimum formal charges.
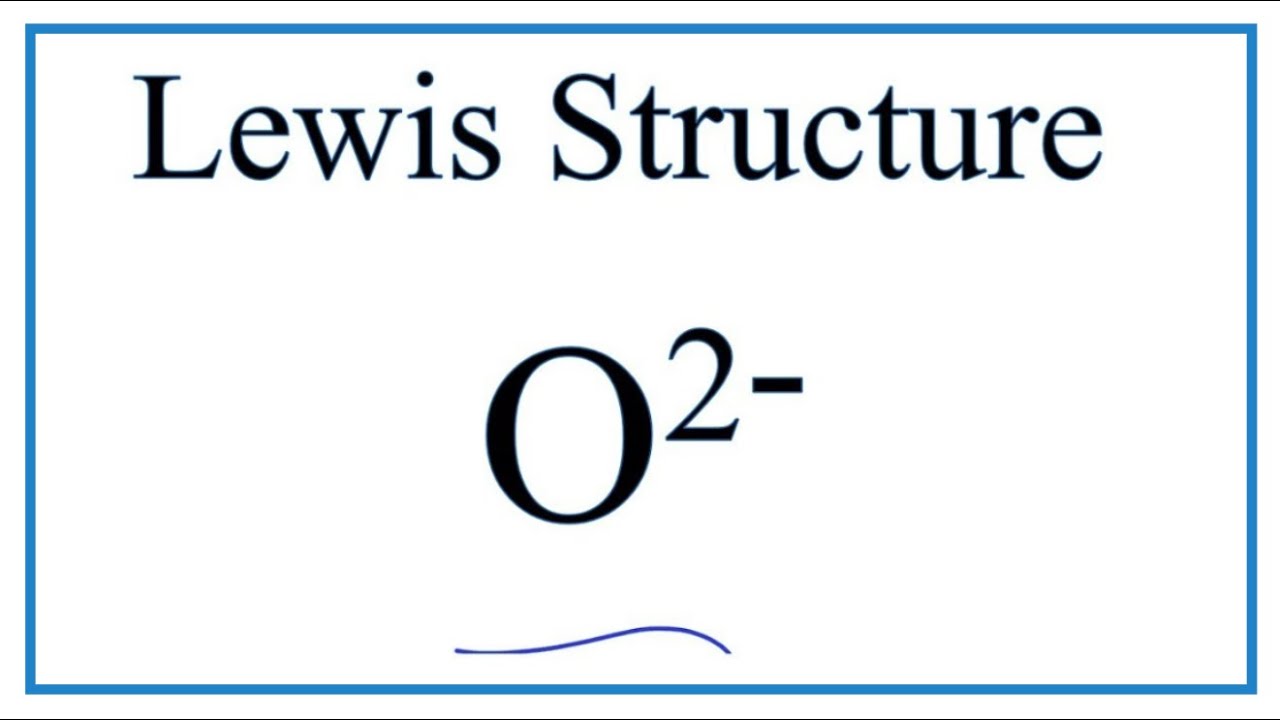
A Lewis dot structure is a drawing of a molecule. The drawing only "works" f0r stable molecules that actually exist. So it's a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram. Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Lewis Dot Diagram For Oxygen. Oxygen has a special rule when doubling. Lewis dot diagrams displaying higher than optimal formal charges represent higher energy states of the species. Lewis dot structures for the first few non-‐metals. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared ... O2 Lewis Structure. O2 Lewis structure, oxygen is the diatomic molecule and hence two atoms of the elements combine together to form dioxygen. The oxygen has six electrons in its valance shell. They try to gain or share two electrons to complete their octet and to get stability. By using the formula of Q, we can calculate the total electrons
Transcript: OK, we're going to do the Lewis dot structure for O2. Let's start. Looking on the periodic table, we can find Oxygen in group 6 or 16, and that means it has 6 valence electrons. But we have two of them so we'll multiply that by 2. That gives us a total of 12 valence electrons. We're going to distribute those valence electrons around the atoms, the Oxygen atoms. So we draw one ...
O2 Lewis structure oxygen electron dot structure is that type of diagram where we show the total 12 valence electrons of O2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots.
Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom. Example A. Determine the total number of valence electrons for C.
Step #3: Draw the Lewis dot diagram. a. Place all the electrons clockwise around the element symbol Reminder: no side receives two dots until each side receives one. Exception: Helium. Step #4: Write down the number of unpaired valence electrons or electrons available for bonding. 5. Which pairs of elements have the same Lewis dot structure?
H2o2 Dot Diagram. The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
O2 Lewis Structure Setup. It’s easiest to think in terms of dots to make the O 2 Lewis structure. Oxygen needs to bond twice, shown as the lone dots on the left and right sides of the oxygen atoms in the below diagram. There are also two pairs of dots, representing four more electrons, that won’t bond. Think of connecting the lone dots to form bonds between each O atom. Each O atom needs ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Diatomic Oxygen).Note that Diatomic Oxygen is often called Molecular Oxygen or just Oxy...
Thus, in its diatomic form by sharing electrons with adjacent oxygen atoms forming a covalent bond it tries to satisfy the octet rule. Then, in the dot ...
The lewis dot structure for o2 or dioxygen is as follows. Ok were going to do the lewis dot structure for o2. Looking on the periodic table we can find oxygen in group 6 or 16 and that means it has 6 valence electrons. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for.
For diatomic oxygen, the Lewis dot structure predicts a double bond. While the Lewis diagram correctly predict that there is a double bond between O atoms, it incorrectly predicts that all the valence electrons are paired ( i.e. , it predicts that each valence electron is in an orbital with another electron of opposite spin).
The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Some elements of the periodic table tend to bond in such a manner that each atom has ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Lewis Dot Structure: The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen.







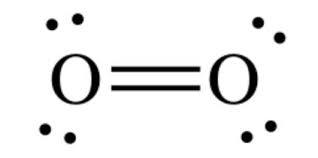
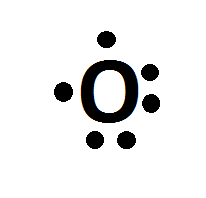












0 Response to "38 o2 lewis dot diagram"
Post a Comment