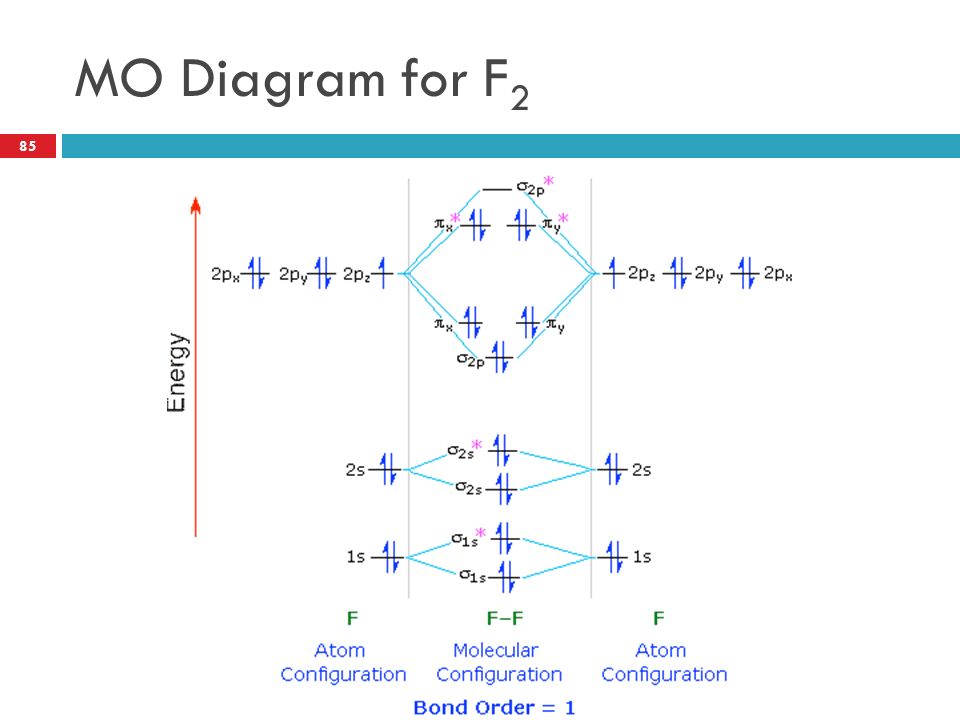
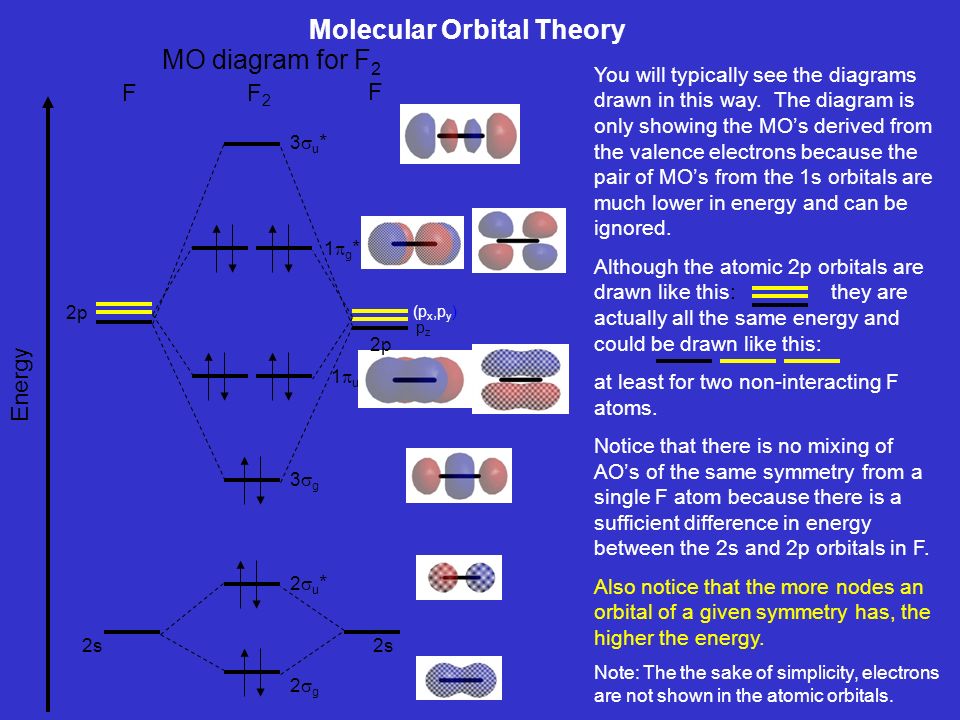
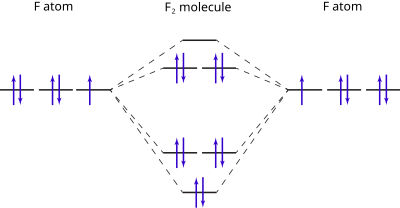
37 molecular orbital diagram for f2
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages Recitation Week 10 (test 3 - Recitation 2) Oct 24, 2016. 1) Draw the molecular orbital diagrams to determine which of the following is most stable. A) F2. B) F2^2+. C) Ne2^2+. D) O2^2+. E) F2^2-. 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. PDF Molecular orbital DiagraM - Magadh University In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! Solution will involve Linear Combination of Atomic Orbitals which are centred around all of the nuclei in molecule, each defined by sets of quantum numbers, with electron probability density determined by ψ 2
What is the difference between bonding and antibonding ... Molecular orbitals are basically mathematical functions that describe the wave nature of electrons in a given molecule. Types According to the molecular orbital theory, there exist three primary types of molecular orbitals that are formed from the linear combination of atomic orbitals. Bonding molecular orbital Anti bonding molecular orbital
Molecular orbital diagram for f2
F2 Molecular Orbital Diagram - 17 images - what is the ... [F2 Molecular Orbital Diagram] - 17 images - programable molecular orbital states of c60 from, no2 molecular orbital diagram untpikapps, what is the energy level diagram of n2 and f2 chemistry, mo bonding in f2 and o2 chemistry libretexts, Li2- Molecular Orbital Diagram The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule .Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2. . , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. . −N a. . ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O.
Molecular orbital diagram for f2. F2 Lewis Structure, Molecular Geometry, Hybridization ... F2 has a molecular weight of 37.997 g/mol. Its boiling point is −188 °C, and its melting point is −219.67 °C. It is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. It is highly reactive, is capable of corroding metals, and is unstable at high temperatures. Cl2 Molecular Orbital Diagram Click here to get an answer to your question how to drew molecular orbital diagram of Cl2. bond order = 1 (like F2) Cl2 has the weakest bond. b. + would be weaker than in Cl2, the Ar-Ar distance would be molecular orbitals in the diagram suggest. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of ... Molecular Orbital Theory - Chemistry This is the molecular orbital diagram for the homonuclear diatomic \ ( {\text {Be}}_ {2} {}^ {\text {+}},\) showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund's rule. Bond Order Answered: The molecular orbital energy diagram… | bartleby The molecular orbital energy diagram for F2 is shown below. Based on this diagram, what is the bond order of F2? o'20 1L 1L 1L1 n2p 1L 1L 1 2p 2p O2p 1L 1L 2s 1 2 3 11 o'is 1L 1L 1s 1s 4 C F Ots 7 8 +/- x 100
Molecular Orbital Diagram For Cl2 - schematron.org Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. 2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals. Draw a molecular orbital diagram of N2 or O2 with magnetic ... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ... Molecular Orbital Theory - Build F2+ - YouTube For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————...
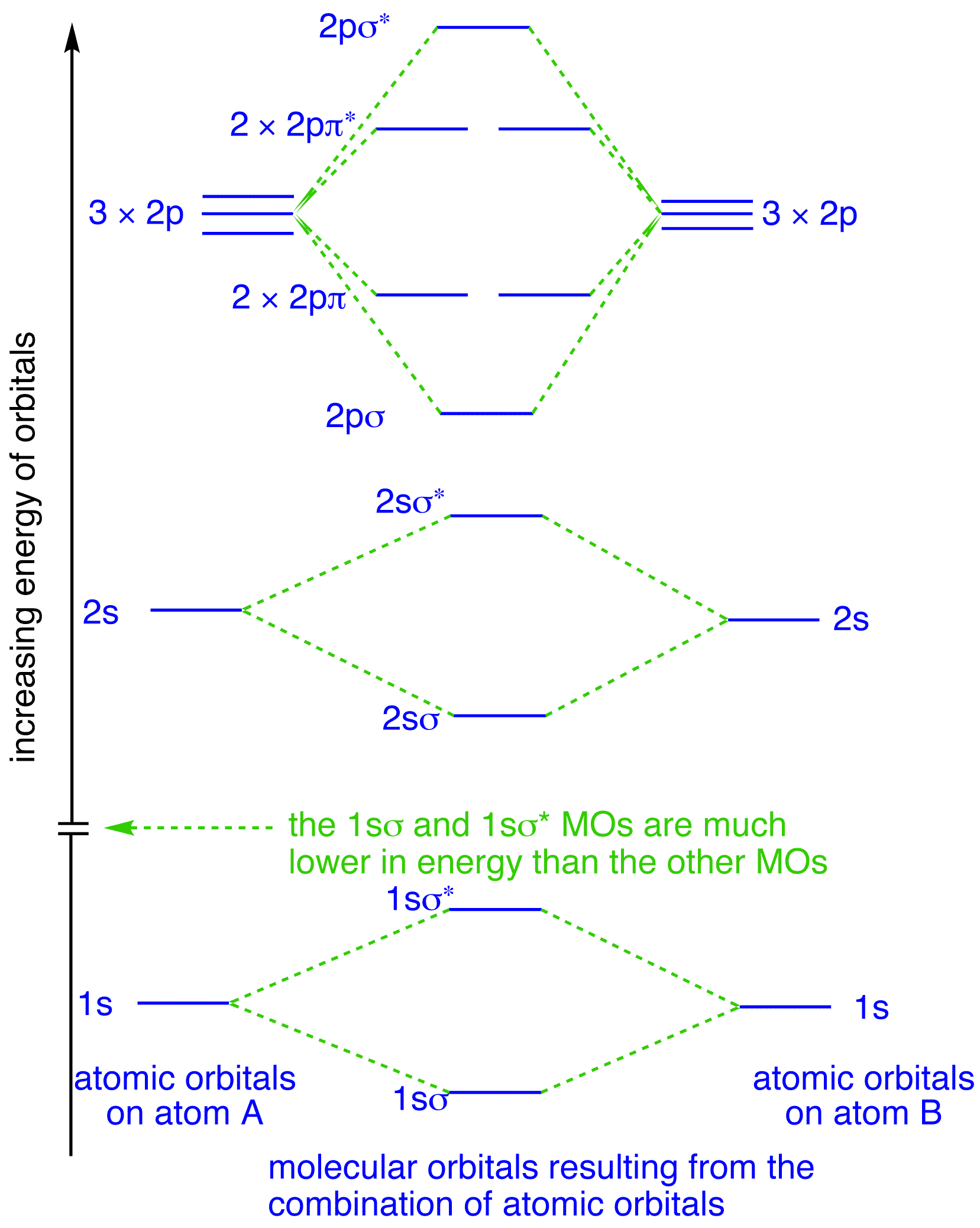
Energy level diagram for Molecular orbitals - Chemical ... The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding electrons Solved Draw the molecular orbital (MO) electron diagram ... Draw the molecular orbital (MO) electron diagram for the F2 molecule. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. Question: Draw the molecular orbital (MO) electron diagram for the F2 molecule. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. What is the molecular electron configuration of "F"_2 ... For example, an ns/ns overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: and an np/np overlap for O2 and F2 gives: So, the full MO diagram is: Thus, the valence electron configuration is: (σ2s)2(σ* 2s)2(σ2pz)2(π2px)2(π2py)2(π* 2px)2(π* 2py)2. Answer link. Molecular Orbitals of Li₂ to F₂ - Chemistry LibreTexts The electronic configuration for F 2 is: s 2s2 s* 2s2 s 2p2 p 2p4 p* 2p4 This electronic configuration shows a single F − F bond in the molecule for the reasons given for the O 2 molecule. The bond lengths and bond energies of O 2 and F 2 (shown on the right) correspond to O = O and F − F respectively.
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Draw molecular orbital diagram for F2 molecule Also class ... N a denotes number of electrons in antibonding molecular orbitals -So, Bond order of F 2 = 8 − 6 2 = 1 -Since, all the electrons in the molecular orbitals are paired, it is diamagnetic. Note: The bonding MO has lower energy and hence greater stability whereas, anti-bonding MO has more energy and hence lesser stability.
What is an (F2-) bond order? - Quora The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is
Draw and write the molecular configuration of nitrogen ... Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Open in App. Solution. Verified by Toppr. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems >
What is the molecular orbital diagram of O2 and F2? - Quora Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2. . , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. . −N a. . ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O.
Li2- Molecular Orbital Diagram The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule .Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
F2 Molecular Orbital Diagram - 17 images - what is the ... [F2 Molecular Orbital Diagram] - 17 images - programable molecular orbital states of c60 from, no2 molecular orbital diagram untpikapps, what is the energy level diagram of n2 and f2 chemistry, mo bonding in f2 and o2 chemistry libretexts,




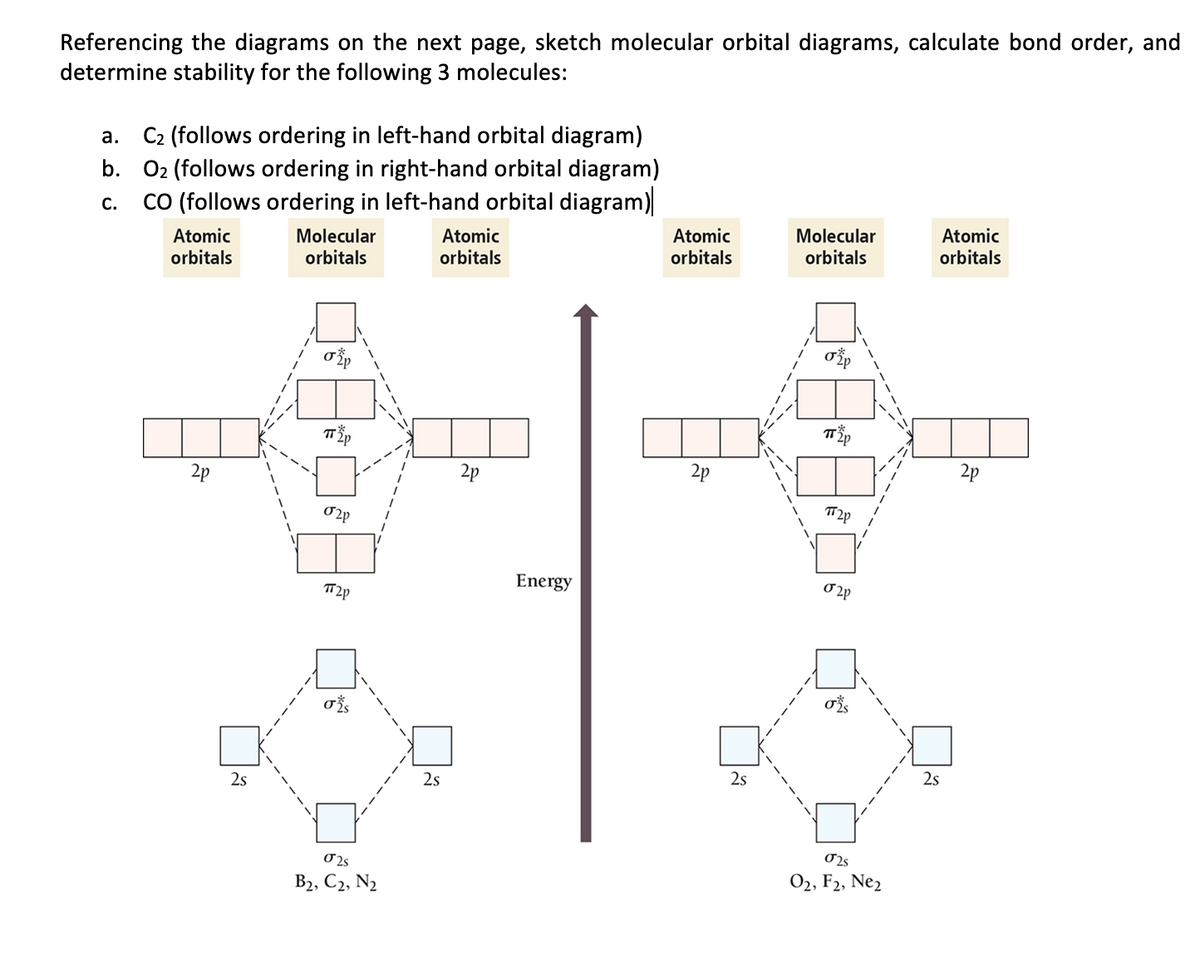
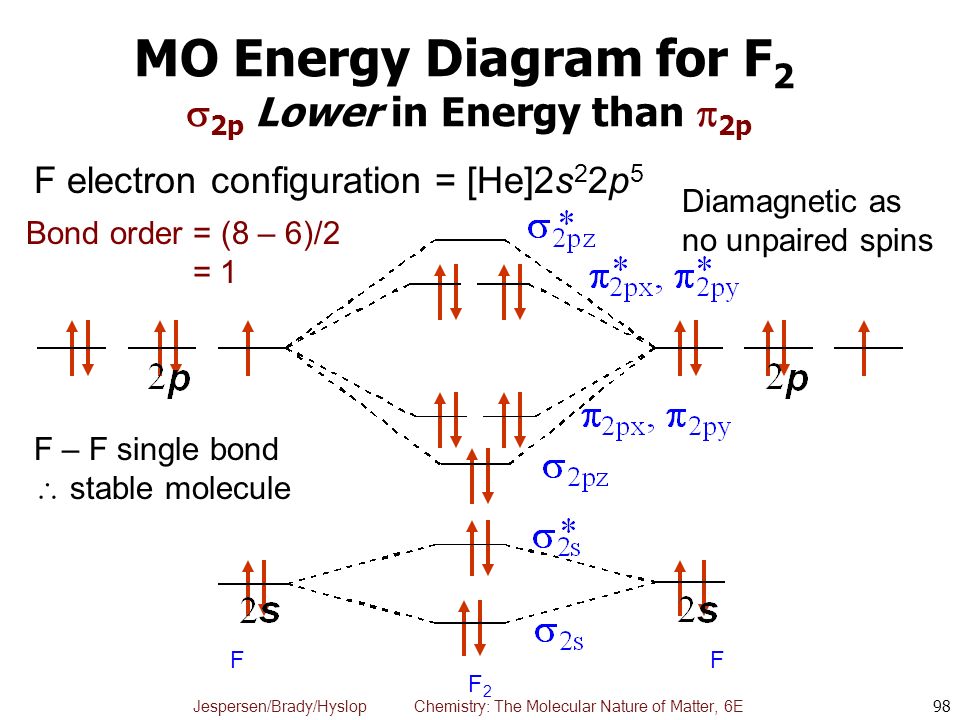
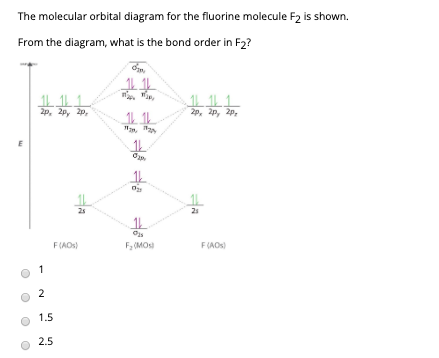

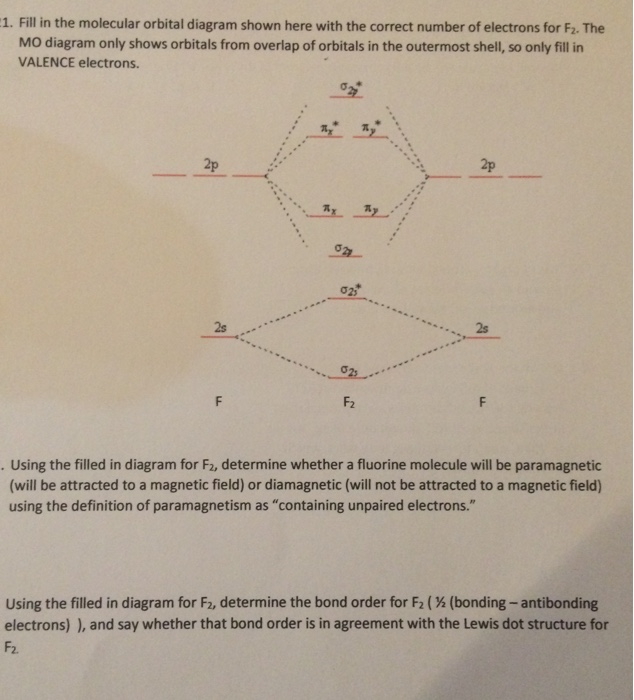







0 Response to "37 molecular orbital diagram for f2"
Post a Comment