38 match the appropriate octahedral crystal-field splitting diagram
Match the appropriate octahedral crystal field splitting diagram. A none of the 3d orbitals point directly at ligands b t2 orbitals are more stable than e orbitals c a small crystal field splitting energy results in a paramagnetic complex d the low spin case gives maximum unpaired electrons e for a given ligand. Solved Match the appropriate octahedral crystal-field | Chegg.com. Science. Chemistry. Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High spin Cr2+ Low spin Fe 2+. Question: Match the appropriate octahedral crystal-field splitting diagram with the given ...
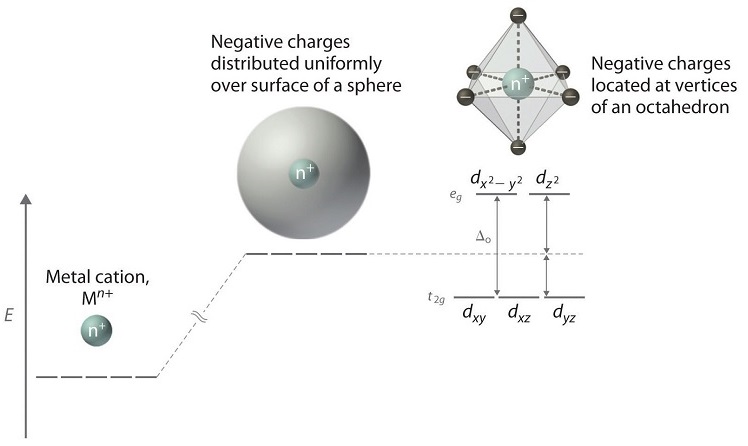
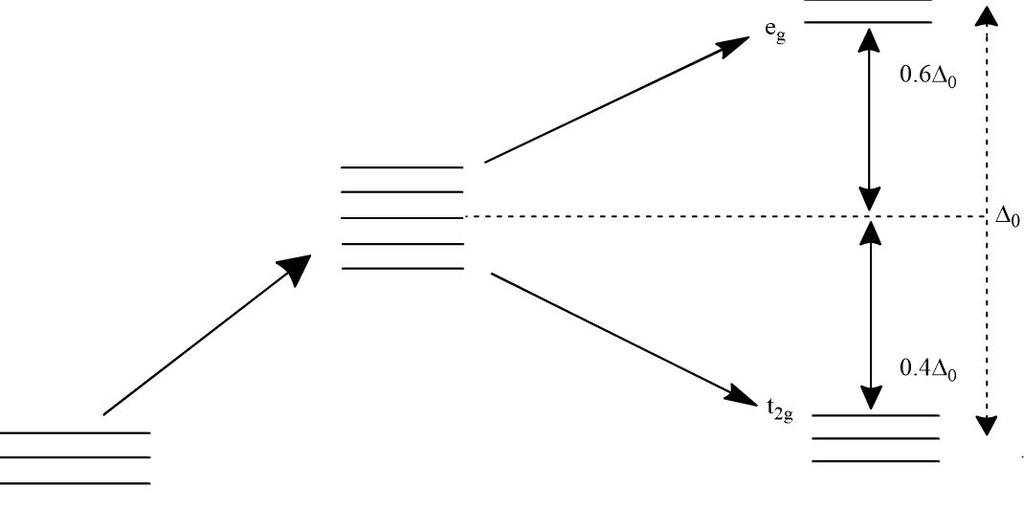
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Match the appropriate octahedral crystal-field splitting diagram
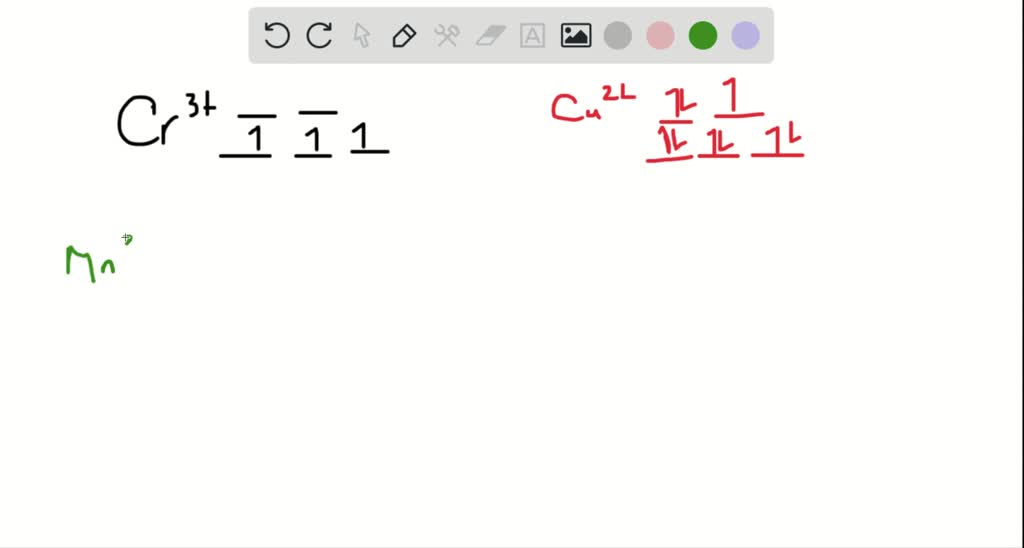
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Co3+ 11 Low-spin Co3+ 1 1 1 1 1L 1 1 1. Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level. This problem has been solved! See the answer. See the answer See the answer done loading. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting DiagramHigh Spin Mn2Low Spin Fe4. Show transcribed image text.
Match the appropriate octahedral crystal-field splitting diagram. Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe3+ low-spin Mn3+ 1 11. Solid State ChemiStry and itS appliCation 2014 Anthony R. West Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High-spin Mn^3+ Low-spin Fe^3+. Sign up to view answer. Our mission is to help you succeed in your Chemistry class. Sign up for free to see the solution.
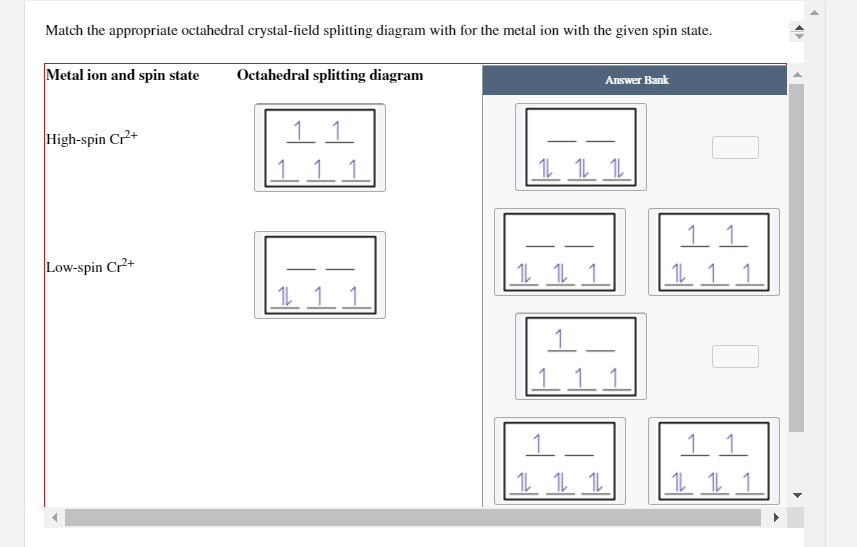
question match the appropriate octahedral crystal field question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion high spin cr 2 low spin fe 3 please give the octahedral splitting diagrams for each ion. crystal field stabilization energy Calculation of the LFSE of tetrachloridocobaltate II. Academia.edu is a platform for academics to share research papers. Academia.edu is a platform for academics to share research papers. Start studying Chemistry. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Mn^3 + Low Spi This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital. As a result the splitting observed in a tetrahedral crystal field ... Explanation:- The valance electron configuration of Fe is 3d⁶4s². So the electron configuration for Fe3+ should be 3d⁵ …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state.

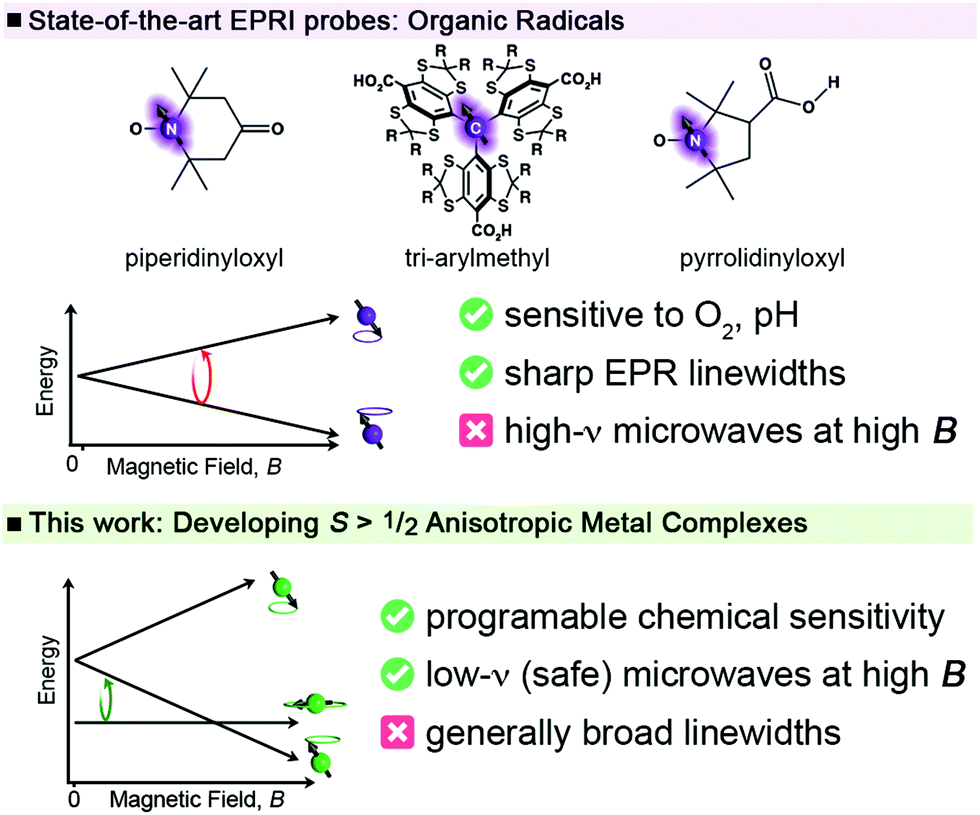
Ligand Control Of Low Frequency Electron Paramagnetic Resonance Linewidth In Cr Iii Complexes Dalton Transactions Rsc Publishing Doi 10 1039 D1dt00066g
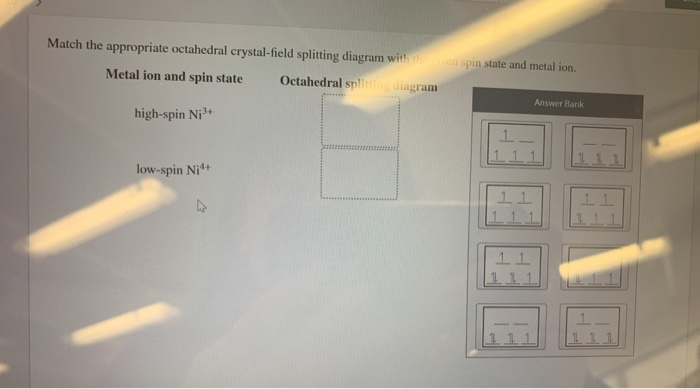
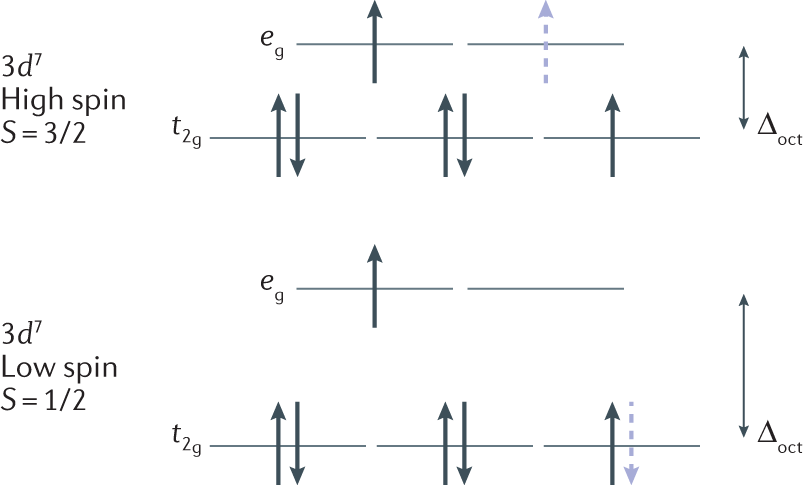
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Ni3+ Low Spin Fe 4+ Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral ...
This problem has been solved! See the answer. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Show transcribed image text.

How Many Unpaired Electrons Would There Be In Each Of The Following Cases Explain A D4 Octahedral Low Spin B D6 Tetrahedral High Spin C D9 Square Planar D D7 Octahedral High
Answer to: Match the appropriate octahedral crystal-field splitting diagram with the given spin stale and metal ion with given spin state. [{Image...
Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Stefanos Mourdikoudis ab, Roger M. Pallares ab and Nguyen T. K. Thanh * ab a Biophysics Group, Department of Physics and Astronomy, University College London, London, WC1E 6BT, UK.
Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. A left handed propeller will pull the stern to starboard right when in reverse. Given this is an octahedral complex your splitting diagram will have 2 degenerate states. Crystal field splitting in an octahedral field eg energy 35 o ...
The shape/ structure of [XeF5]– and XeO3F2, respectively, are : [Main Sep. 02, 2020 (II)] (a) pentagonal planar and trigonal bipyramidal (b) octahedral and square pyramidal (c) trigonal bipyramidal and pentagonal planar (d) trigonal bipyramidal and trigonal bipyramidal 5. The molecular geometry of SF6 is octahedral.
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+.
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Fe^3+ Low Spin Co^2+
The transition metals, groups 3–12 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. (Although the metals of group 12 do not have partially filled d shells, their chemistry is similar in many ways to that of the preceding groups, and we therefore include them in our discussion.)
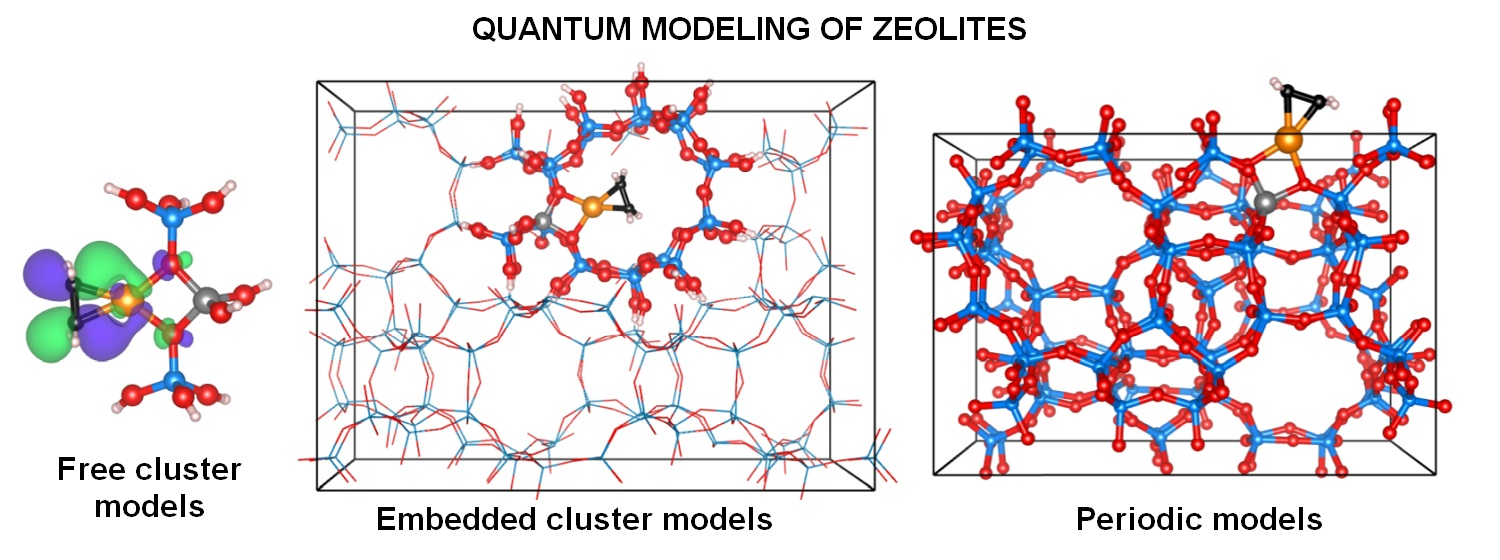
Molecules Free Full Text Zeolites At The Molecular Level What Can Be Learned From Molecular Modeling Html
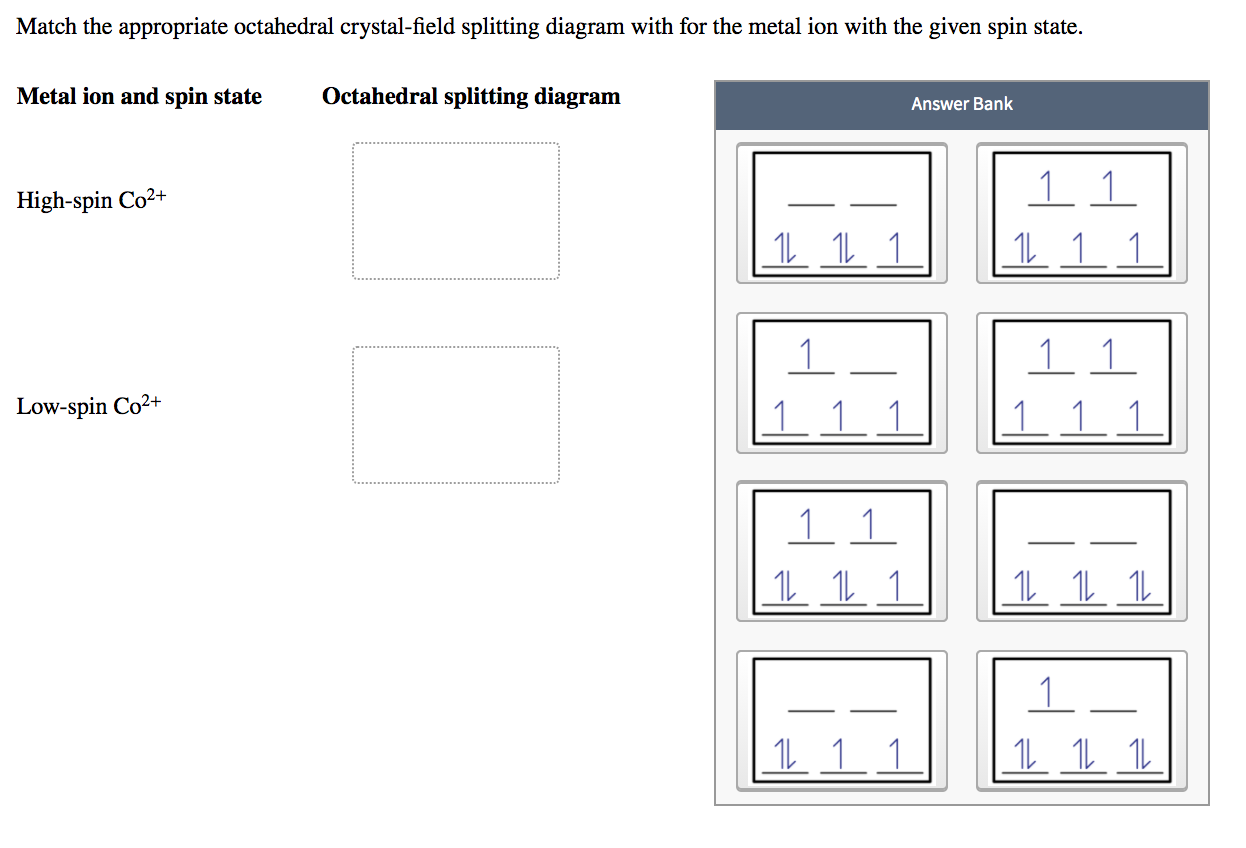
Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Co2+ 1 1 1 1 1 1 1 1 1 1 1 1 1 1 Low-spin Co2+ 1 1 1 1 1 11 11 11 1 1 1 1 1
Match the Column 3 Chapter 1. Solid State Q. Match the items of column A to those given in column B. Column A Column B 1. Colour in crystals Tetrahedral arrangement of atoms 2. Diamond F-centre 3. Hexagonal close packing Co-ordination number of 8 4. CsCl crystal BCC 5. 68% occupancy of space ABAB type of close packing 6. Metallic crystal Diamond 7.
This problem has been solved! See the answer. See the answer See the answer done loading. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting DiagramHigh Spin Mn2Low Spin Fe4. Show transcribed image text.
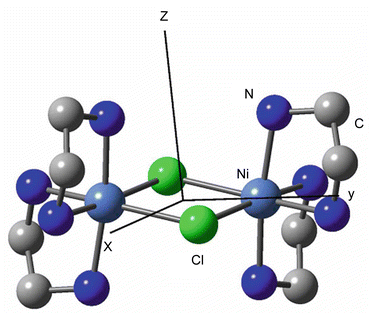
Zero Field Splitting In Transition Metal Complexes Ab Initio Calculations Effective Hamiltonians Model Hamiltonians And Crystal Field Models Springerlink
Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Co3+ 11 Low-spin Co3+ 1 1 1 1 1L 1 1 1.
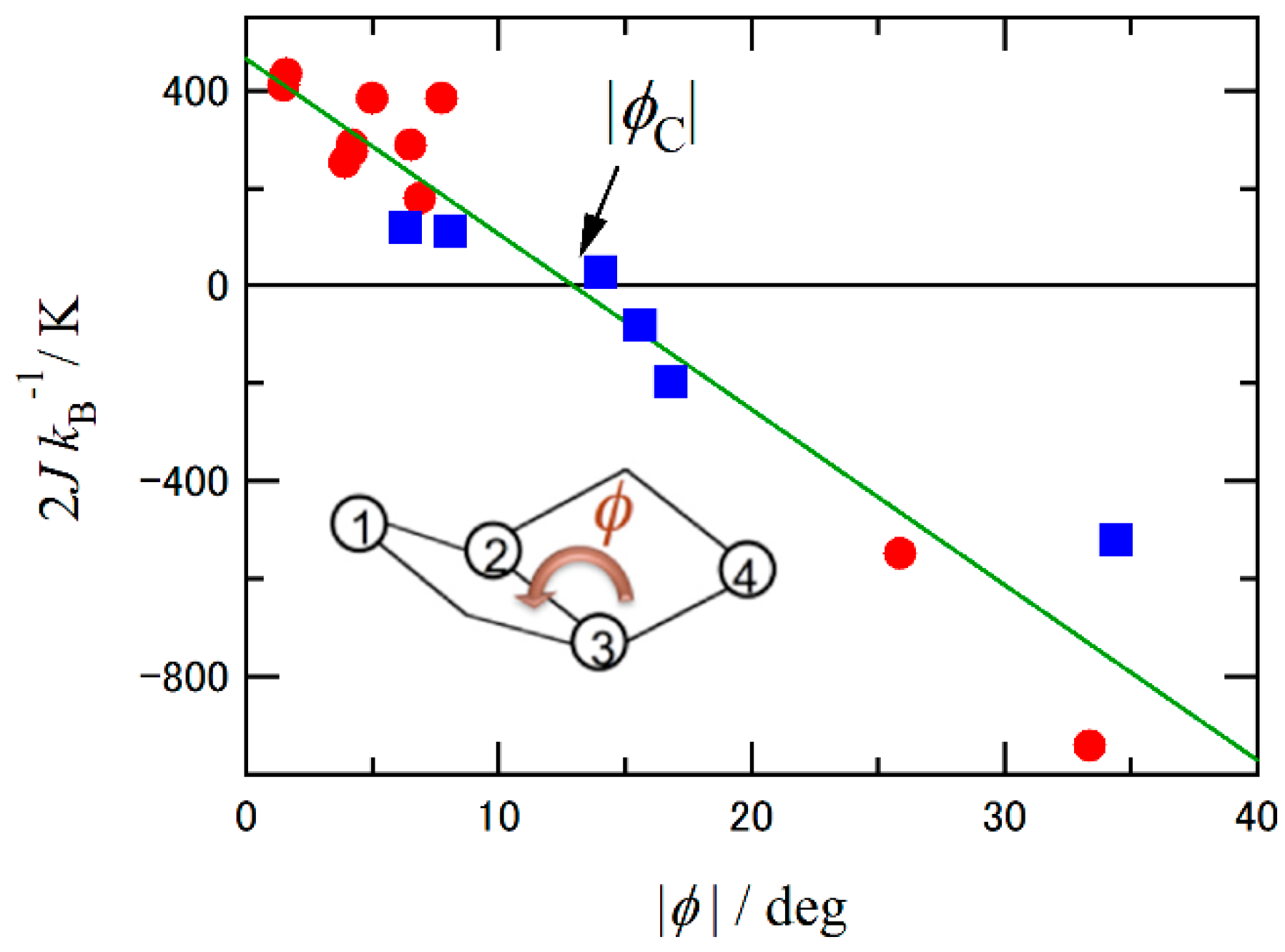
Inorganics Free Full Text Molecular S 2 High Spin S 0 Low Spin And S 0 2 Spin Transition Crossover Nickel Ii Bis Nitroxide Coordination Compounds Html

Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin State And Metal Ion Metal Homeworklib

Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin State And Metal Ion Metal Homeworklib

A Theoretical Perspective On Charge Separation And Transfer In Metal Oxide Photocatalysts For Water Splitting Zhou 2019 Chemcatchem Wiley Online Library














0 Response to "38 match the appropriate octahedral crystal-field splitting diagram"
Post a Comment