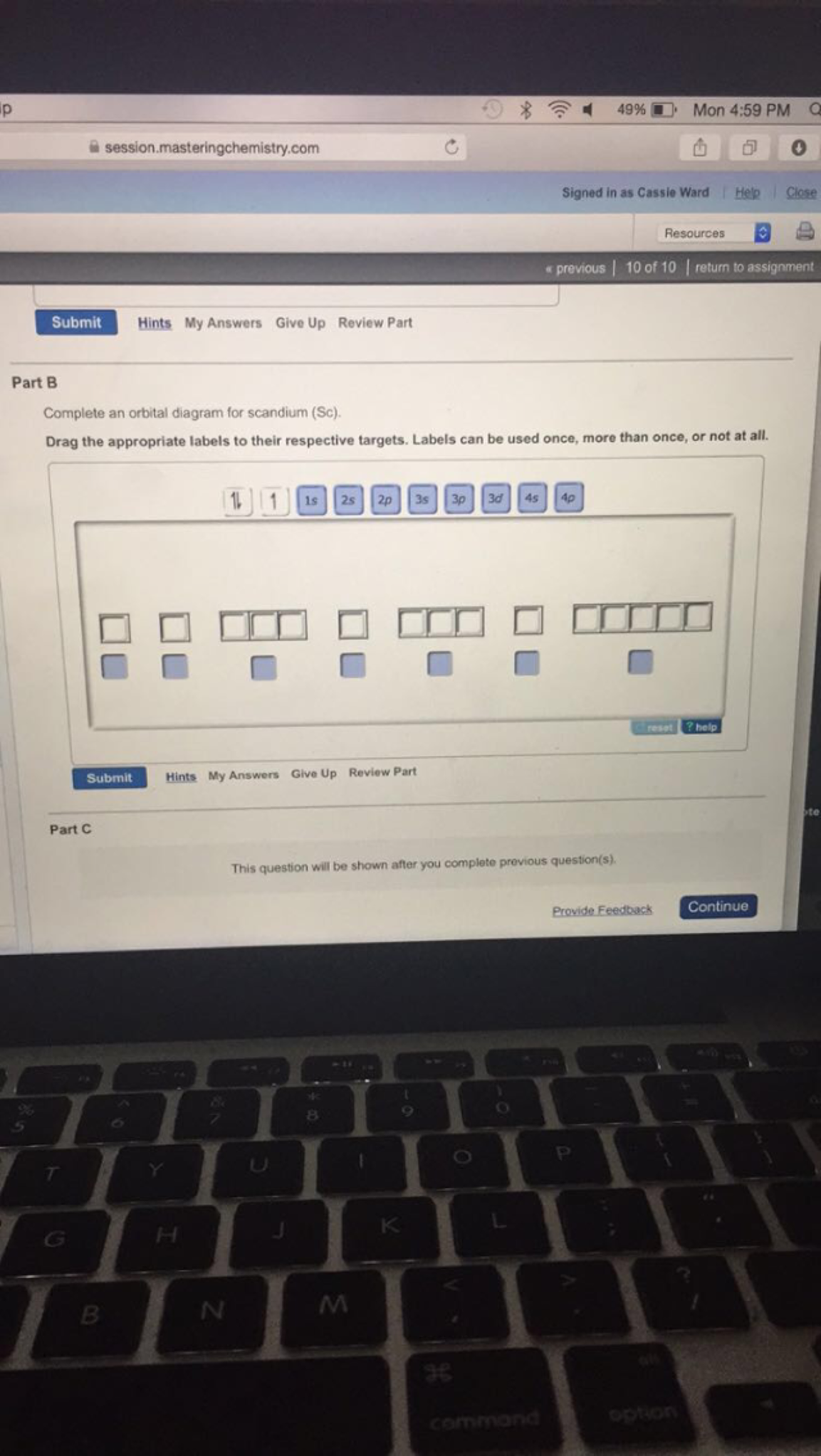
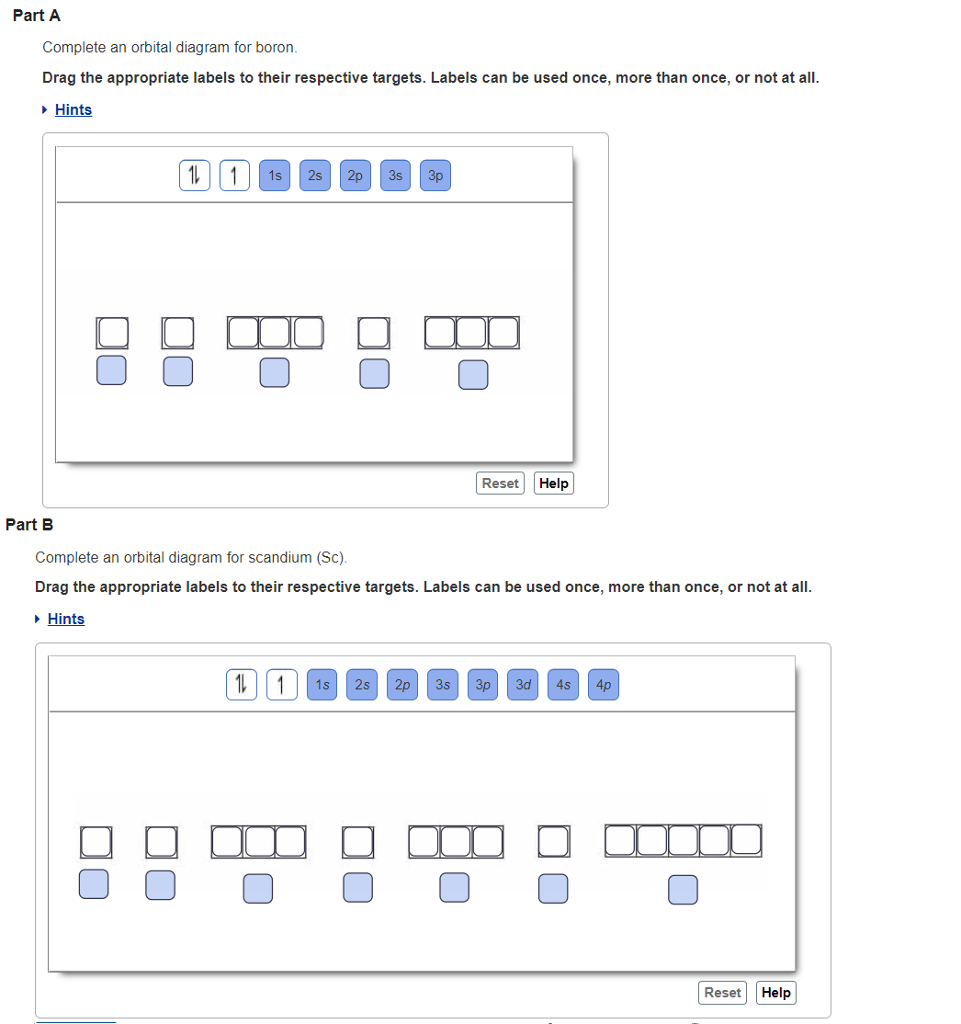
37 complete an orbital diagram for scandium (sc).
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. SHOW ANSWER. assuming that your periodic table has eighteen columns, you should highlight the four elements in the third column from the left of the table. explanation. neutral atoms of elements after calcium contain d electrons. the energy of d orbitals of a particular main shell lies between the energy of the s and p orbitals of the next main ...
Nov 01, 2021 · Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc ...

Complete an orbital diagram for scandium (sc).
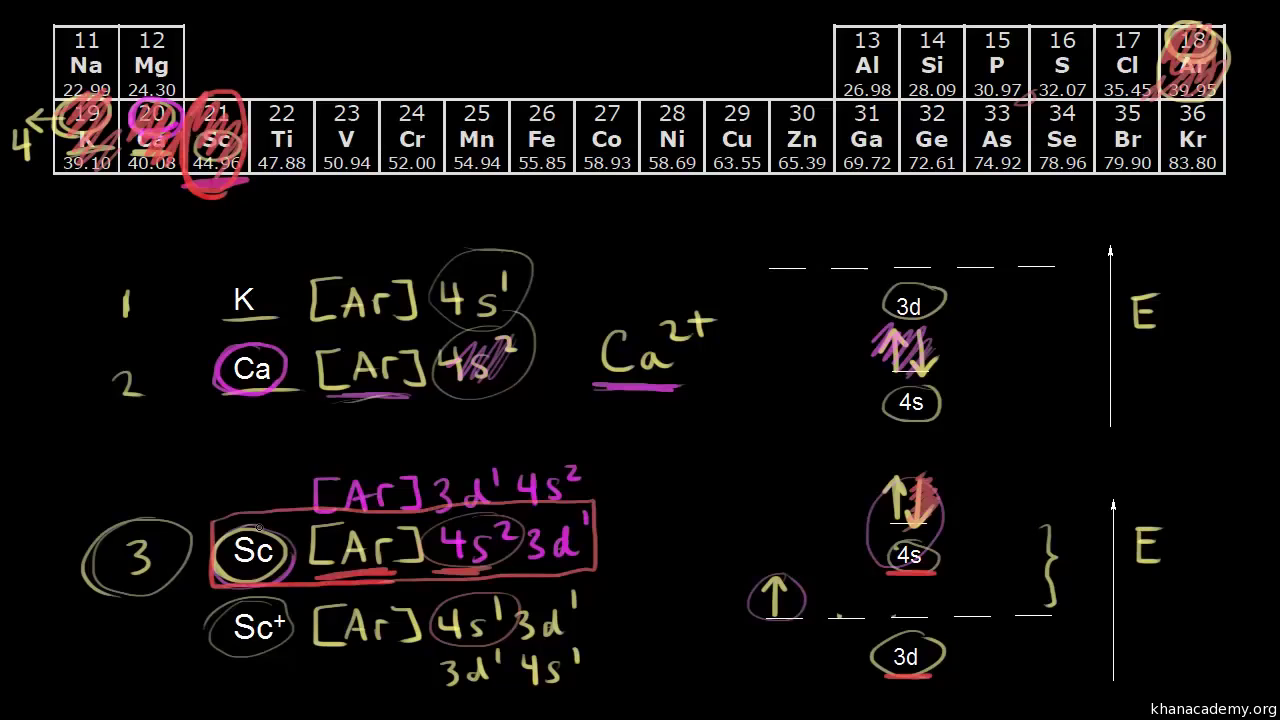
S u n C o u n tr y A i r l i n e s H o l d i n g s , I n c Common Stock The se lling stockholder i dentified in this prospectus is selling 7,250,000 shares of common stock of Sun Country Airlines Holdings, Inc, a Delaware corporation We are not selling any shares of our common stock, and we will not receive any of the proceeds from the saleA s us e d i n t hi s A nnua l R e port on F orm 10K ... Chapter 9 Electrons in Atoms and the Periodic Table. 9.1 True/False Questions. 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.
Complete an orbital diagram for scandium (sc).. Answer (1 of 7): The diatomic molecules having less than or equal to 14 electrons in all show s-p mixing. Eg:- N_2,CO,C_2,BN, etc. In such molecules, the energy difference between 2s and 2p orbitals is quite less and due to it the 2s orbital and 2p_z orbital tend to overlap. The element scandium - one of the so-called transition metals - follows calcium in the periodic table. Scandium has nine (9) electrons in the 3n electron shell. Of these, two are accommodated in the 3s orbital and six more in the 3p orbitals. The ninth electron occupies a d orbital. This is where things start to get really interesting. About Xplore<br><br>Xplore is an early stage Seattle-based company offering Space as a Service®. Xplore provides hosted payloads, communication relay services and exclusive datasets to its customers via the Xcraft®, the company's multi-mission spacecraft. Xplore's mission is to expand robotic exploration via commercial missions at and beyond Earth. We are a small company, growing rapidly ... Nov 01, 2021 · Valence Electrons of First 30 elements (List+Shell Diagram) November 1, 2021 October 22, 2020 by Admin Valence electrons of first 30 elements of the Periodic table are mentioned below.
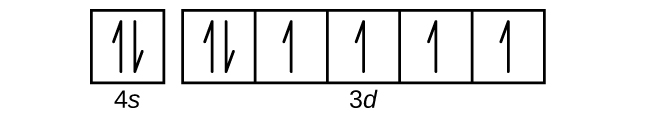
Metallic scandium was produced for the first time in 1937. The first pound of 99% pure scandium metal was produced in 1960. Production of aluminum-scandium alloys began in 1971 following a U.S. patent. Aluminum-scandium alloys were also developed in the USSR. Aug 14, 2020 · The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals. Chapter 9 Electrons in Atoms and the Periodic Table. 9.1 True/False Questions. 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter.
S u n C o u n tr y A i r l i n e s H o l d i n g s , I n c Common Stock The se lling stockholder i dentified in this prospectus is selling 7,250,000 shares of common stock of Sun Country Airlines Holdings, Inc, a Delaware corporation We are not selling any shares of our common stock, and we will not receive any of the proceeds from the saleA s us e d i n t hi s A nnua l R e port on F orm 10K ...

Write The Shorthand Electron Configuration For Each Of The Following For Each Configuration 1 Indicate The Core Electrons 2 The Outer Electrons And 3 Draw The Electron Orbital Diagram For The Outer
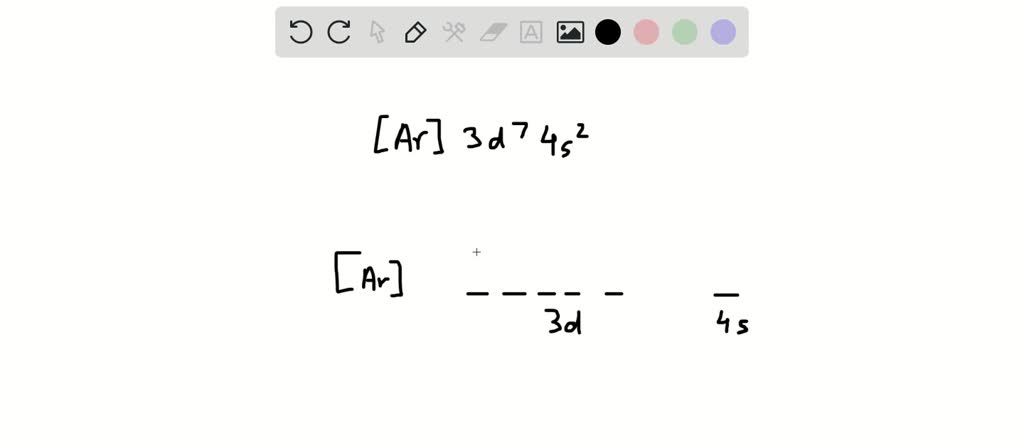
Solved Write The Full Form Electron Configuration With The Orbital Diagram For Scandium Sc What Are The Quantum Number Values N Mi M For The 9th Electron In Scandium Arrange The Following In
Solved Consider The Element Scandium Complete The Table Below By Providing A Set Of Quantum Numbers For The Last Two Electrons In A Scandium Atom Course Hero

No Office Hours Today What S Coming Up Oct 25the Atmosphere Part 1ch 8 Oct 27midterm No Lecture Oct 29the Atmosphere Part 2ch 8 Nov 1light Ppt Download

Sc Scandium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
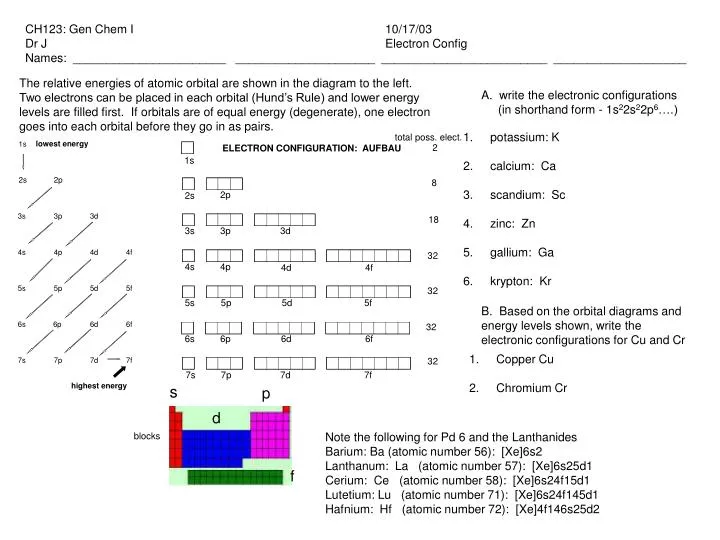
Ppt Potassium K Calcium Ca Scandium Sc Zinc Zn Gallium Ga Krypton Kr Powerpoint Presentation Id 3222214

Complete Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configuration Of Valence Homeworklib
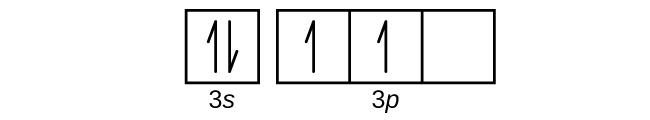




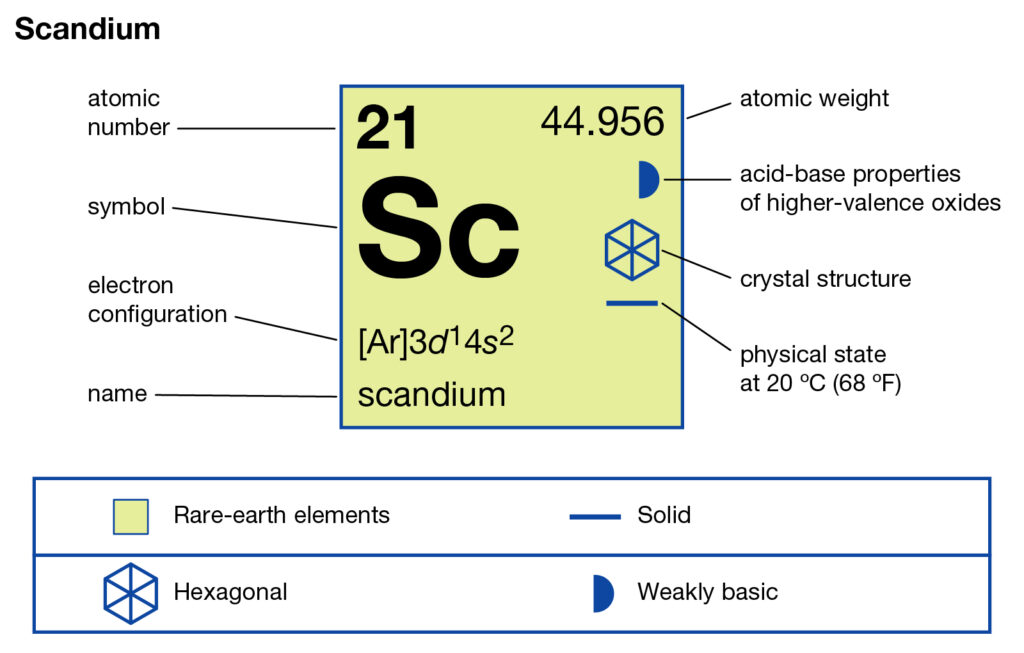













0 Response to "37 complete an orbital diagram for scandium (sc)."
Post a Comment