38 lewis dot diagram for ccl4
CCl4 Lewis Structure|| Lewis Structure for CCl4 (Carbon ... CCl4 Lewis Structure|| Lewis Structure for CCl4 (Carbon Tetrachloride)||Which is the correct Lewis structure for carbon tetrachloride CCl4?||How do you draw... CCl4 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc...
Nitrobenzene | C6H5NO2 - PubChem Nitrobenzene is an industrial chemical. It is an oily yellow liquid with an almond-like odor. It dissolves only slightly in water and will evaporate to air. It …
Lewis dot diagram for ccl4
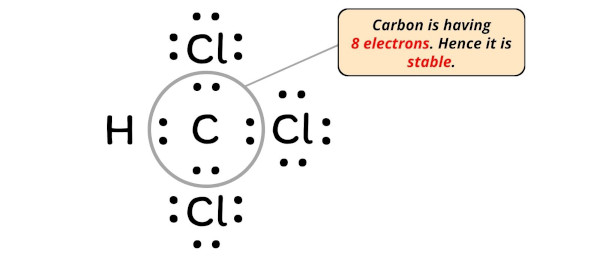
How to draw a CCl4 Lewis Structure? - Science Education ... In a CCl4 Lewis Structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CCl4 Lewis Structure, with all four Chlorines arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for C CCl4 Lewis Structure, Molecular Geometry, Hybridization ... CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether. 1,2-Dichloroethane | ClCH2CH2Cl - PubChem Ethylene Dichloride is a clear, colorless, oily, synthetic, flammable liquid chlorinated hydrocarbon with a pleasant chloroform-like smell that emits toxic fumes of hydrochloric acid when heated to decomposition. Ethylene dichloride is primarily used to produce vinyl chloride.Inhalation exposure to this substance induces respiratory distress, nausea and vomiting and affects the central …
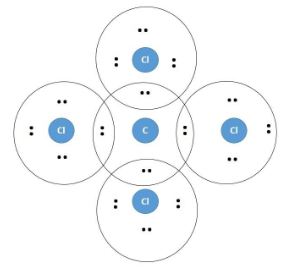
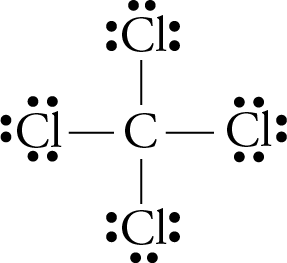
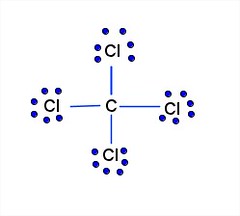
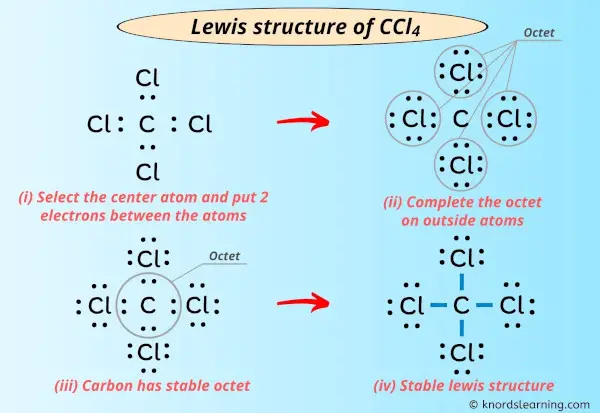
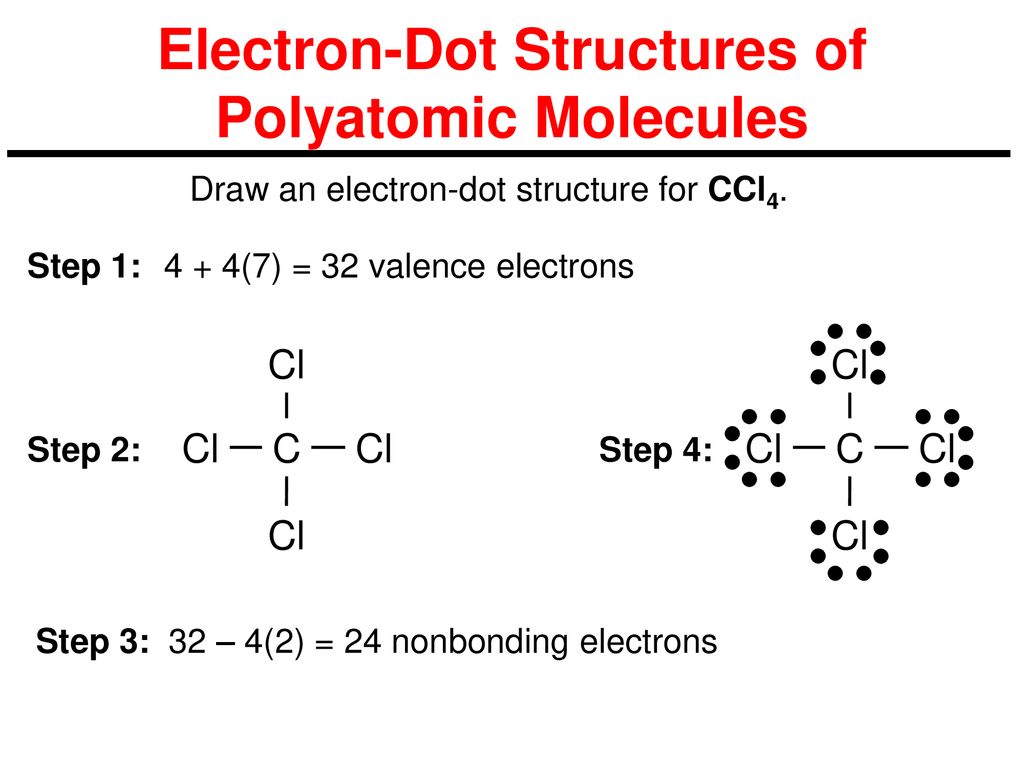
Lewis dot diagram for ccl4. Draw electron dot representation of CCl4 class 11 ... Hint: A Lewis electron dot diagram is a representation of the valence shell electrons of an atom that uses dots around the symbol of the central element as a representation of the electrons.The bonds are represented as pairs of electrons shared. Complete Stepwise solution In carbon tetrachloride or $ \text{CC}{{\text{l}}_{\text{4}}} $ , the central element is carbon, whose valence shell has ... C2h2 Lewis Dot Structure - what is the lewis dot structure ... C2h2 Lewis Dot Structure - 18 images - c2h2 lewis structure valence electrons formal charge, draw the molecules include all lone pairs of electrons, 10 best lewis dot structures images on pinterest drawing, nl 9849 dot diagram of co2 free diagram, BIOLOGY J - Essay Help 2022-03-07 · Examine the diagram of RBCs which exhibit ABO antigens on their surface, in the left box of figure 2 below. Using the legend in the right box, determine the blood type of the cells in the left box… Deduce the genotype of members of the Kim family who were not tested (Akari, Aki’s mother, and Yunseo, Aki’s other sister). Punnett Squares are provided below. If … Carbon tetrabromide (CBr4) lewis dot structure, molecular ... The lewis structure of CBr4 is similar to CCl4 and CF4, since, they all are in the same group in the periodic table and contain the same number of valence electrons. Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4. Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis …
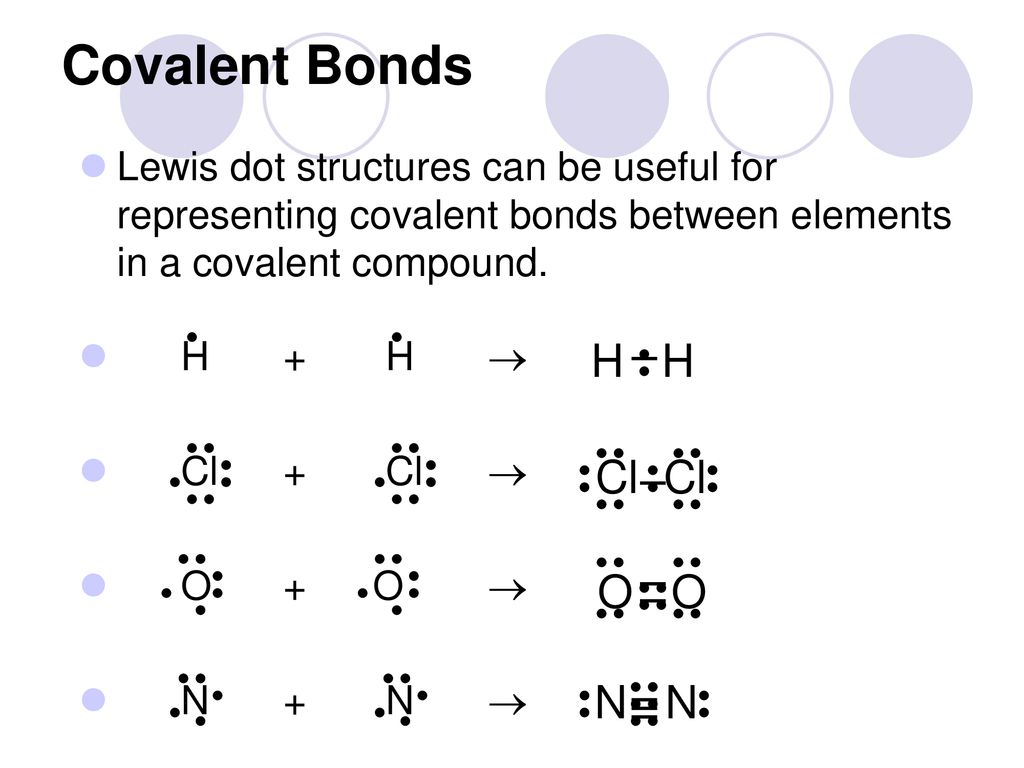
Organic Chemistry Questions and Answers | Study.com Draw the product formed for each of the following chemical reactions, using either line structures, condensed formulas, or Lewis structures. If you use condensed formulas or … Lewis Dot Structure of CCl4 (Carbon TetraChloride) - YouTube I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle. What is the molecular geometry of CCl4? Draw its VSEPR and ... The Lewis structure shows that there are four electron regions about the central carbon atom. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. For these clouds in CCl4 to be as far as possible from one another, they will point toward the corners of a tetrahedron. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
What is the Lewis dot structure for CCl4? - Studyrankersonline 982 views. asked Dec 11, 2019 in Important Questions by megha00 Expert (17.8k points) What is the Lewis dot structure for CCl4? Draw the Lewis Structure of CCl4 - Nassau Community College 1) Draw the Lewis Structure of CCl4. What is the electron geometry of the central C atom? What is the molecular geometry of the central C atom? What is the hybridization of the central C atom? What is the molecular geometry or shape of the molecule? Does CCl4 have a dipole moment? 2) Draw the Lewis Structure of H2S. SiO2 Lewis Structure, Molecular Geometry, Hybridization ... 2022-04-16 · This is the last step in completing the Lewis diagram for SiO2. As we all know, silicon needs 8 electrons to complete its octet, but it only has 4 electrons right now. By turning an oxygen atom’s electrons into a covalent bond, we would be able to achieve the octet of silicon. We convert two lone pairs of electrons from each oxygen atom to a covalent bond, as seen in … (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Gallery of solved draw the lewis dot structure for each of ... Solved Draw The Lewis Dot Structure For Each Of The images that posted in this website was uploaded by Media.nbcmontana.com. Solved Draw The Lewis Dot Structure For Each Of The equipped with a HD resolution 2224 x 2718.You can save Solved Draw The Lewis Dot Structure For Each Of The for free to your devices.. If you want to Save Solved Draw The Lewis Dot Structure For Each Of The with original ...
Carbon tetrachloride Lewis structure? - Answers Carbon tetrachloride has the molecular formula of CCl4. It is comprised of a single carbon (C) and four chlorine (Cl) atoms. The Lewis dot structure for CCl4 is: ..
Lewis structure for CCl4? - Answers The Lewis dot structure for CCl4 starts with a C in the middle. Four dashes are drawn, one on each side, each connecting to a Cl atom. On the unconnected sides of the Cl atoms, there are two dots ...
CCl4 Lewis Structure - How to Draw the Dot Structure for ... Let's do the Lewis structure for CCl4, Carbon Tetrachloride, sometimes just called Carbon Tet. We'll start by looking at the valence electrons. Carbon is in group 4 or 14, so it has 4. Chlorine has 7 valence electrons, but we have 4 Chlorines so let's multiply that by 4. Four plus 28 equals 32 total valence electrons to work with.
Pearson Iit Foundation Series - Chemistry Class 9 By ... Homogeneous Stainless steel, brass and bronze NaCl in water and iodine in CCl4 Alcohol and water and benzene and toluene Liquor ammonia and soda water Air – Heterogeneous Iron and sulphur Sulphur in water Oil and water – – Hydrogen gas adsorbed on Pd Example “All pure substances are homogenous. But, all homogenous substances are not pure”. Justify solution A …
CCl4 Lewis Structure - Science Trends A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral ...
quizlet.com › 567217475 › chemistey-ex-2-flash-cardsChemistey Ex 2 Flashcards | Quizlet A Lewis structure includes all the valence electrons in the species. Select all statements that correctly describe formal charge. Formal charge can be used to determine the most plausible Lewis structure for a given compound.
Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane Because of this symmetrical geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds, fats and oils. It can also dissolve iodine. It is somewhat volatile, giving off vapors having a smell characteristic of other chlorinated ...
Lewis Dot Structure and Polarity of CCl4 (Carbon ... Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. It is an inorganic compound that is non-flammable. This ScienceStruck post provides you with the Lewis dot structure diagram and the polarity of carbon tetrachloride.
idealcalculator.com › lewis-structure-calculatorLewis structure calculator | Lewis structure generator Lewis structures can be used to represent valence shell electrons in a chemical bond. The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species.
CCL4 Molecular Geometry, Lewis Structure, Hybridization ... Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let's calculate the total valence electrons.
ekc.reitausbildung-reese.de › poenwekc.reitausbildung-reese.de email protected] [email protected]
(PDF) Inorganic Chemistry 4th edition ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft
CCl4 Lewis Structure ||Lewis Dot Structure for CCl4 ... CCl4 Lewis Structure ||Lewis Dot Structure for CCl4||Carbon Tetrachloride Lewis Structure#CCl4LewisStructure#LewisStructureforCCl4This video has solved the f...
1,2-Dichloroethane | ClCH2CH2Cl - PubChem Ethylene Dichloride is a clear, colorless, oily, synthetic, flammable liquid chlorinated hydrocarbon with a pleasant chloroform-like smell that emits toxic fumes of hydrochloric acid when heated to decomposition. Ethylene dichloride is primarily used to produce vinyl chloride.Inhalation exposure to this substance induces respiratory distress, nausea and vomiting and affects the central …
CCl4 Lewis Structure, Molecular Geometry, Hybridization ... CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether.
How to draw a CCl4 Lewis Structure? - Science Education ... In a CCl4 Lewis Structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CCl4 Lewis Structure, with all four Chlorines arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for C


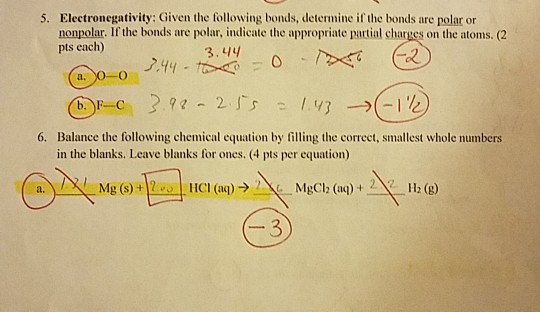


























0 Response to "38 lewis dot diagram for ccl4"
Post a Comment