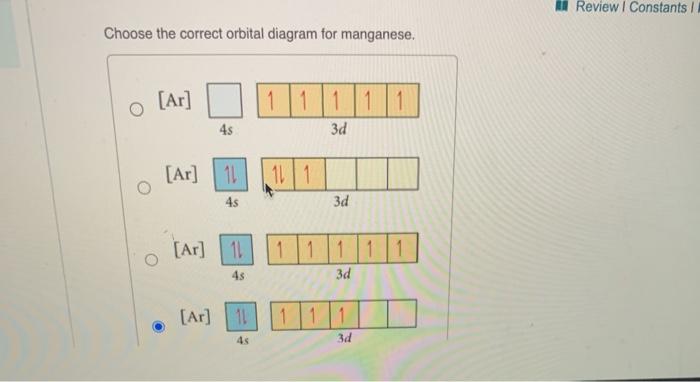
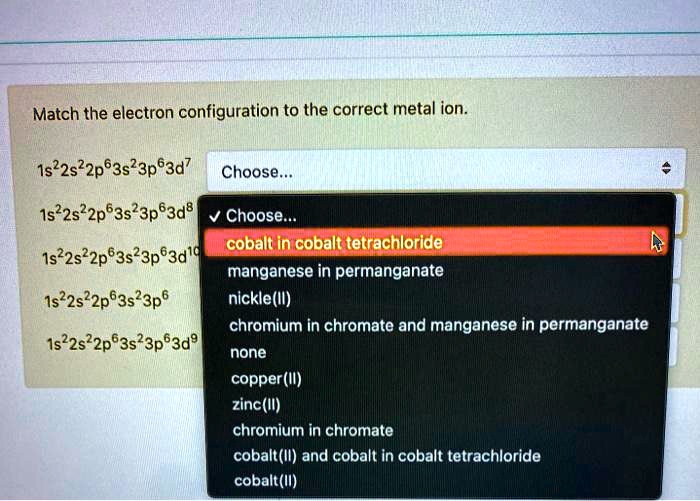
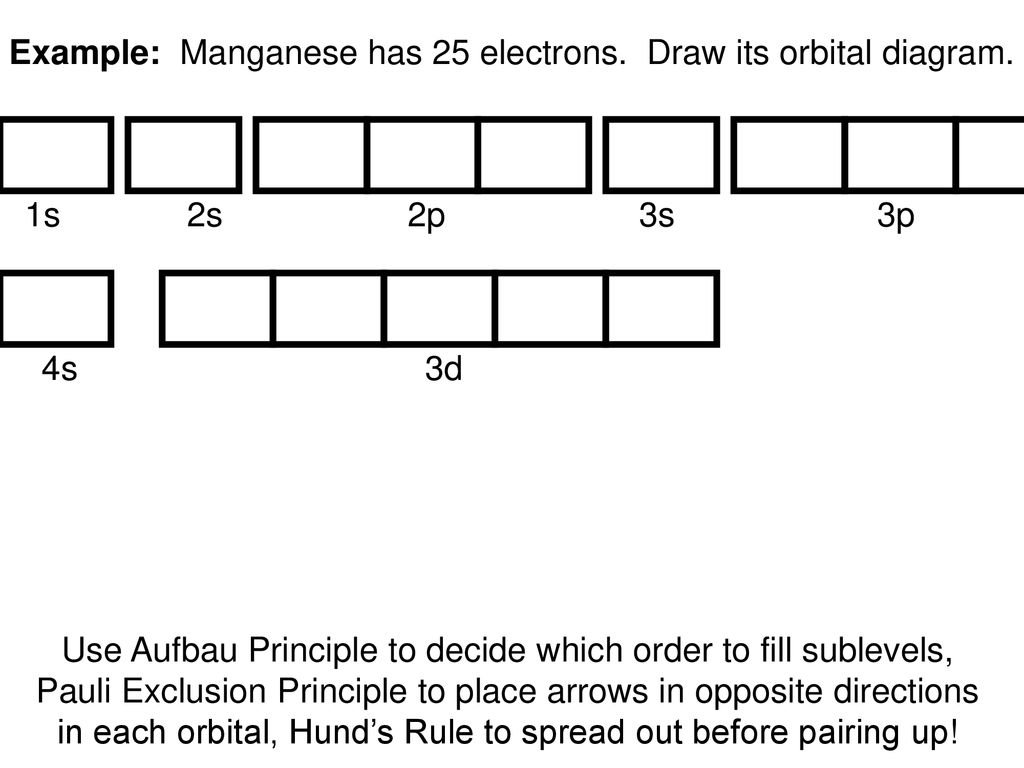
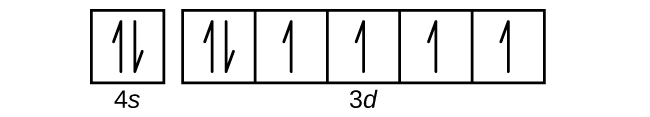
39 choose the correct orbital diagram for manganese.
Electron Configuration Chart of All Elements (Full Chart) Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). Orbital Diagram For Vanadium (V) | Vanadium Electron ... Vanadium Electron Configuration of V will be written as: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3. Therefore many users will not be able to remember it and some will be those who will find it very difficult to write it. Therefore there is one more way that you can learn the electronic configuration and that is [Ar] 3d3 4s2.
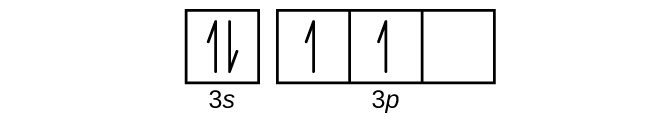
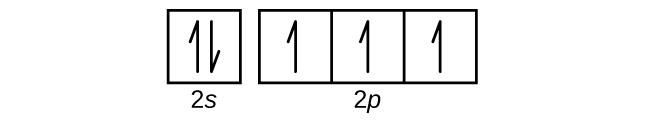
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. Now the users can get a good idea about how to write the electronic configuration of the element.

Choose the correct orbital diagram for manganese.
What is the electronic configuration of Mn3+? | Socratic The electron configuration for a "Mn"^(3+)" ion is "[Ar]3d"^4". Manganese has atomic number 25, meaning its atoms have 25 protons in their nuclei. A neutral manganese atom also has 25 electrons. However, the manganese 3+ ion, "Mn"^(3+)", has 22 electrons. This gives it 3 more protons than electrons, which gives it the 3+ charge. The electron configuration in noble gas shorthand for a neutral ... 5.3 Electron Configuration Flashcards - Quizlet The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p (Get Answer) - Choose the correct orbital diagram for ... A student draws the following orbital diagram for vanadium. What kind of feedback would you provide - select from the list below (you may select more than one answer). a. Orbital diagram obeys the Aufbau principle. b.The noble gas chosen for...
Choose the correct orbital diagram for manganese.. ⚗️what is the orbital diagram for manganese? - Brainly.com Find an answer to your question what is the orbital diagram for manganese? saragrady saragrady 02/25/2021 Chemistry High School answered What is the orbital diagram for manganese? 2 See answers 40 choose the correct orbital diagram for vanadium ... 37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3. 39) The element manganese (symbol = Mn) has five valence electrons. What is the orbital diagram for manganese? Explanation: Oxidation States. +2,3,4,6,7. Electrons Per Shell 2 8 15. Electron Configuration [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5 Chemistry Chapter 4 and 5 - Subjecto.com Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge.
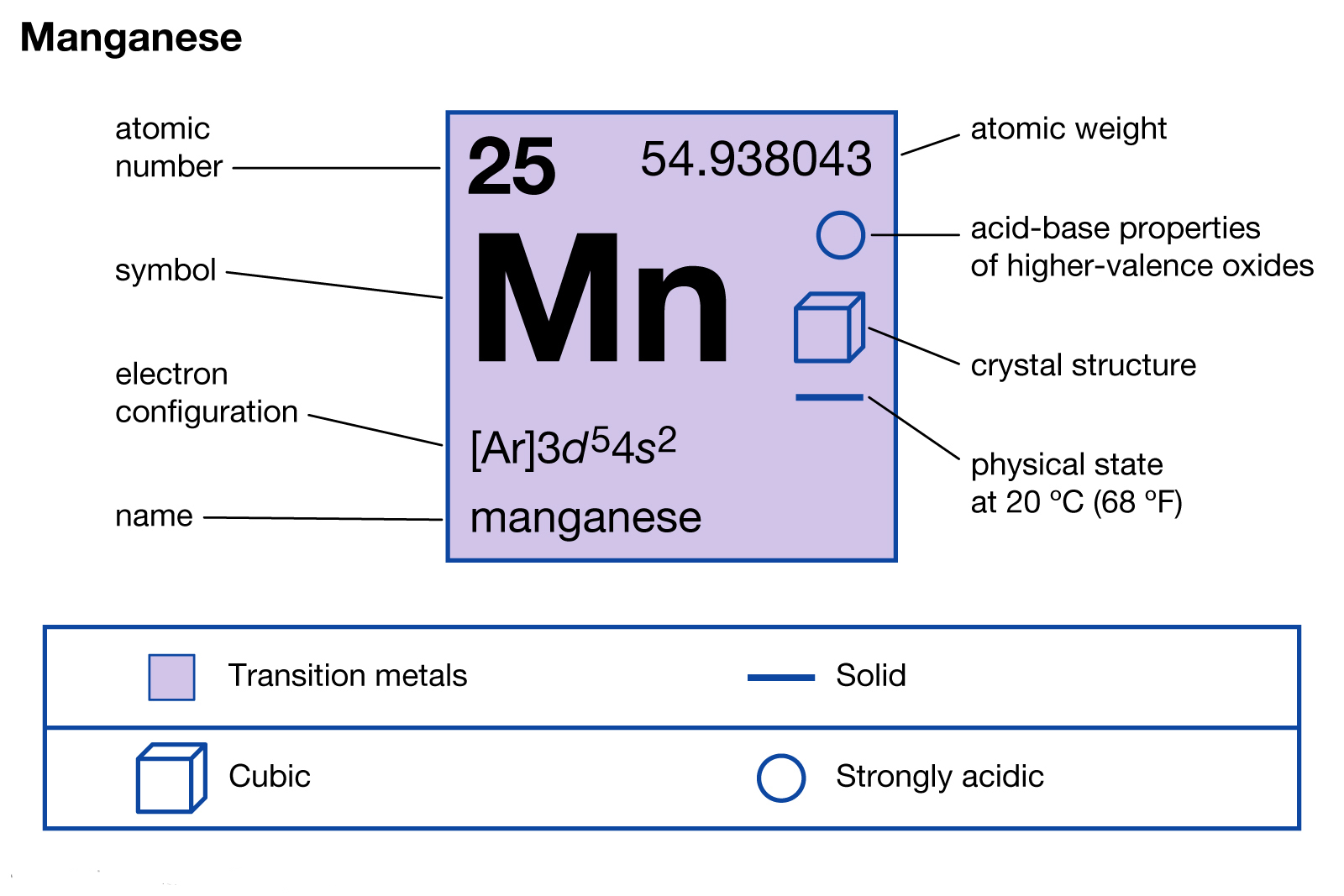
PDF Chemistry Practice Test Periodic Trends and Orbitals Periodic Trends and Orbitals MULTIPLE CHOICE. Choose the one alternative that ... The correct electron configuration for manganese is: A) 1s2 2s22p63s23p64s23d5 B)1s22s22p63s23p64s23d104p1 C) 1s22s22p63s23p6 ... Diagram I B) Diagram II C) Diagram IV D) Diagram III ... How many orbitals does manganese have? - Pvillage.org III. 4s electrons contribute to the manganese covalent bonding, while 3d electrons do not. What is the symbol of manganese? Mn Manganese/Symbol. Which is the correct orbital diagram for manganese? The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . What are the quantum numbers of magnesium? Chem lecture: Ch.8 Quiz Questions Flashcards | Quizlet Choose the statement that is TRUE. *Core electrons effectively shield outer electrons from nuclear charge. *Core electrons are the easiest of all electrons to remove. *Outer electrons efficiently shield one another from nuclear charge. *Valence electrons are most difficult of all electrons to remove. *All of the above are true. Electron Configuration for Magnesium (Mg) Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
Solved stion 15 Part A Which is the correct orbital ... stion 15 Part A Which is the correct orbital diagram for manganese? 3d Submit Request Answer MW. Answered: What is the correct orbital diagram for… | bartleby Solution for What is the correct orbital diagram for the 3d electrons in Fe? Chemical Bonding Quiz | Chemistry Quiz - Quizizz answer choices. Oxygen bonds randomly with elements in unpredictable ways. Electronegativity differences between the bonding elements allow electrons to be either shared or donated. The difference in atomic radii between the groups accounts for the difference in behavior. PDF Electron Configurations and Orbital Diagrams key The 3p electrons are in three different orbitals and have the same spin. 4. Use the periodic table to identify the neutral atoms having the following electron configurations: Electron Configuration Element [Ne] 3s 2 magnesium [Ar] 4s 2 3d 5 manganese [Kr] 5s 2 4d 10 5p 3 antimony 5. -3Consider the following ions: N , O -2, F -1, Na +1, Mg +2, and Al+3. a.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11
stjon 15 Part A Which is the correct orbital diagram for ... hi there for this problem. We are asked to identify the correct orbital diagram Formal Injun, um, in its ground state. So I want thio just to get us started here. I want to write out the electron configuration from lived in, um, lived in, um, it's atomic number 42. Therefore, it needs 42 electrons using the shorthand method. I am going to go to the period before we find before where we find ...
orbital diagram for manganese Latest Information | Echemi Echemi shares information about orbital diagram for manganese. Our coverage of orbital diagram for manganese news, knowledge and opinion is widely.
Solved Review I Constants / Choose the correct orbital ... Question: Review I Constants / Choose the correct orbital diagram for manganese, O [Ar] 11 1 1 3d 45 [Ar] 11 111 4s 3d [Ar] 11 1 1 1 1 1 3d 4s [Ar] 11 111 3d This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (4 ratings)
What is the orbital notation for manganese? - Answers Wiki User. ∙ 2015-06-01 20:20:24. Study now. See answer (1) Best Answer. Copy. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 is the orbital notation formanganese. Wiki User. ∙ 2015-06-01 20:20:24.
The manganate and permanganate ions are tetrahedral, due to Click here👆to get an answer to your question ️ Question The manganate and permanganate ions are tetrahedral, due to: A. The T-bonding involves overlap of p- orbitals of oxygen with d-orbitals of manganese B. There is no 1-bonding C. The n-bonding involves overlap of p- orbitals of oxygen with p-orbitals of manganese D. The n-bonding involves overlap of d- orbitals of oxygen with d ...
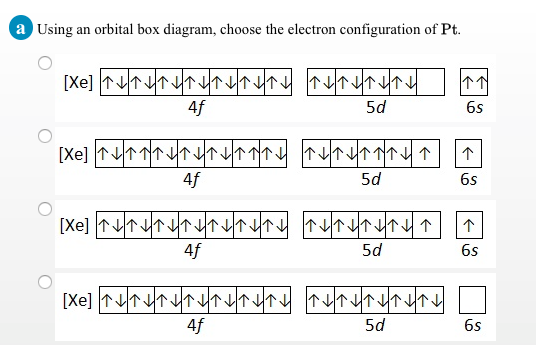
SOLVED:Choose the correct orbital diagram for the ground ... hi there. In this problem, we want to write out some electron configurations and draw some orbital diagrams for four specific elements. And while we're doing t…
What Is the Electron Configuration of Selenium? Sub-shells s, p, d and f hold a maximum of two, six, 10 and 14 electrons, respectively. In selenium, the first energy level has two electrons in sub-shell s. The second energy level holds eight electrons. Two of those electrons are in sub-shell s, while the other six are found in sub-shell p. The third energy level has a total of 18 electrons.
(Get Answer) - Choose the correct orbital diagram for ... A student draws the following orbital diagram for vanadium. What kind of feedback would you provide - select from the list below (you may select more than one answer). a. Orbital diagram obeys the Aufbau principle. b.The noble gas chosen for...
5.3 Electron Configuration Flashcards - Quizlet The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
What is the electronic configuration of Mn3+? | Socratic The electron configuration for a "Mn"^(3+)" ion is "[Ar]3d"^4". Manganese has atomic number 25, meaning its atoms have 25 protons in their nuclei. A neutral manganese atom also has 25 electrons. However, the manganese 3+ ion, "Mn"^(3+)", has 22 electrons. This gives it 3 more protons than electrons, which gives it the 3+ charge. The electron configuration in noble gas shorthand for a neutral ...






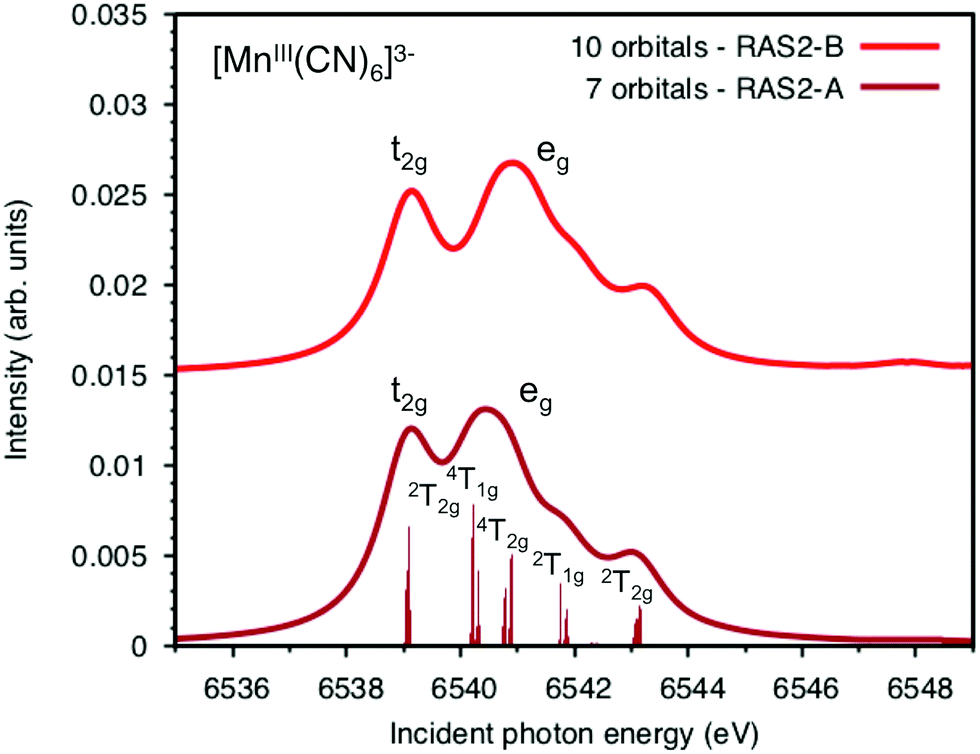











![Pick out the correct statement with respect to [Mn(CN)6]^3 - :](https://d1hj4to4g9ba46.cloudfront.net/questions/675758_638303_ans_71f2a26841d34892832142a31c2effbe.png)




0 Response to "39 choose the correct orbital diagram for manganese."
Post a Comment