37 orbital diagram of f-
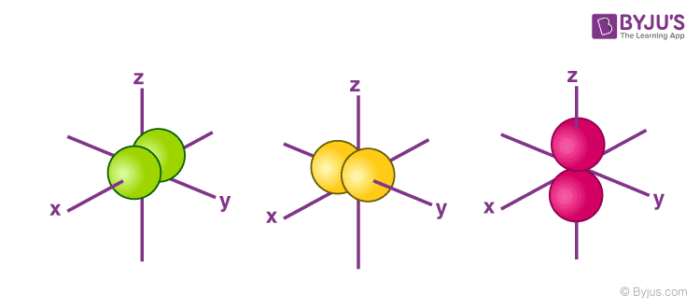
Atomic orbital - Wikipedia The shapes of the first five atomic orbitals are: 1s, 2s, 2p x, 2p y, and 2p z. The two colors show the phase or sign of the wave function in each region. Each picture is domain coloring of a ψ (x, y, z) function which depend on the coordinates of one electron. Solved Use the molecular orbital energy diagram below to ... Use the molecular orbital energy diagram below to answer the questions about bond order for the molecule Nz. Number of Number of Bonding electrons Antibonding electrons N2 Bond Order This corresponds to: A. Single bond B. Double bond c. Triple bond D. Half of a bond E. Between a single and double bond F. Between a double and a triple bond G.
S P D F orbitals Explained - 4 Quantum Numbers, Electron ... This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
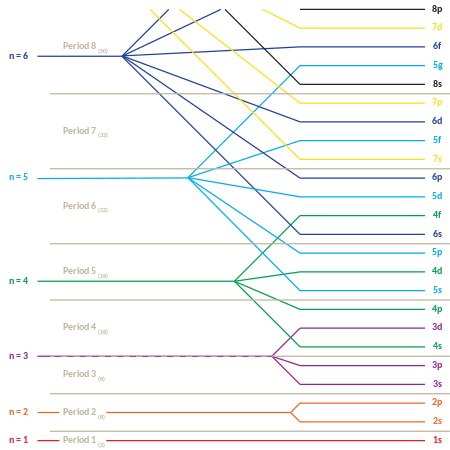
Orbital diagram of f-
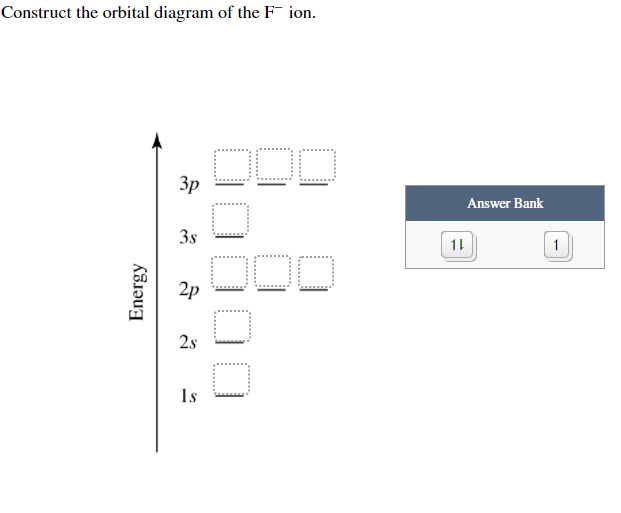
Solved Construct the orbital diagram of the F- ion ... This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F- ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (24 ratings) F Orbital Shape - EWT F Orbital. The sequence for the f block is unique. Beginning with lanthanum (Z=57) it starts a block that contains 15 elements. The 5 th level of a tetrahedron has 15 units. There are 15 elements for the f block (Z=57 to 71), although an odd number affects the number of orbitals (14 / 2 = 7). It converts a proton to neutron in the next d block to compensate, beginning with the 5d block. PDF Instructions Discussion Problem The F MO Diagram Instructions: (Part 2) Complete the F 2 orbital interaction diagram below. The 8 MOs should be labeled and ordered correctly relative to one another. Absolute position however is not expected. Draw dashed lines to show which atomic orbitals interact to form the MOs.
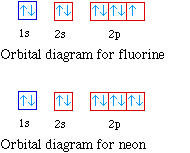
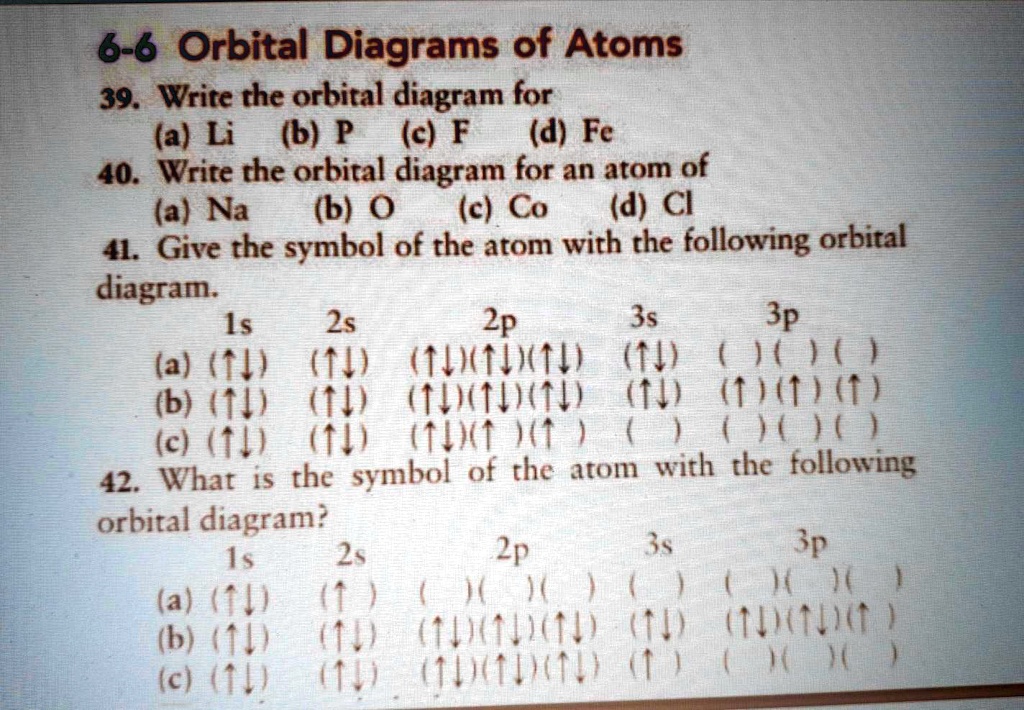
Orbital diagram of f-. How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ... Construct the orbital diagram of the f ion - Soetrust Construct the orbital diagram of the f ion. By soetrust March 29, 2022. Gamers!!! Amazon Luna launches with freebies for Prime subscribers. Amazon Luna special offer for Prime members! Fluorine (F) Orbital diagram, Electron configuration, and ... The orbital diagram of Fluorine contains 1s orbital, 2s orbital, and 2p orbital. 1s orbital contains 1 box, 2s orbital also contains 1 box and 2p orbital contains 3 boxes. Fluorine has a total of 9 electrons and one box can hold up to the two electrons. Construct the orbital diagram of the f ion Jan 03, 2022 · The Fluoride ion formed by addition of electron to its neutral state. F + e^- rightarrow F^- Thus, F^- ion has 10 electrons. The electronic configuration of Fluoride ion is 1s^2 3s^2 2p^6. As the energy of the atomic orbital is 1s^2 < 2p^6 (2p^2_x = 2p^2_y = 2p^2_z), the orbital energy diagram is represented as shown below:
️Molecular Orbital Diagram Practice Worksheet Free ... Molecular orbital diagram practice worksheet The f 2s is nonbonding. This will give trigonal bipyramidal geometry and is called dsp3 hybridization. Answers to practice test questions 3. For each of the following molecules, draw the lewis diagram and tally up the electron pairs. Get 30 Molecular Orbital Diagram Practice Worksheet Wiring Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... What is the electron configuration of F^-? | Socratic Jun 23, 2016 · F: 1s22s22p5 Now, the F− anion is formed when 1 electron is added to a neutral fluorine atom. Notice that the 2p-subshell of the neutral atom contains 5 electrons. Its maximum capacity is actually 6 electrons, two electrons for each p-orbital. This means that the extra electron will be added to one of the three 2p-orbitals, let's say to 2py. Orbitals Chemistry (Shapes of Atomic Orbitals) - Shape of ... The orbital wave function or ϕ is a mathematical function used for representing the coordinates of an electron. The square of the orbital wave function or represents the probability of finding an electron. This wave function also helps us in drawing boundary surface diagrams.
Solved Construct the orbital diagram of the F^- ion. A ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. Atomic Orbitals Definition, Shapes, Examples And Diagrams These are s, p, d and f. The shapes of these orbitals are discussed below: s-orbitals. The s-orbitals are solid spherical shape around the nucleus. When principal quantum number n = 1 and azimuthal quantum number l = 0, that is 1s orbital which is closest to the nucleus. When n = 2 and l = 0 , i.e 2s orbital which contains one node. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). s,p,d,f Orbitals - Chemistry | Socratic The 3dx² - y² orbital looks exactly like the first group, except that that the lobes are pointing along the x and y axes, not between them. The 3dz² looks like a p orbital wearing a doughnut around its waist. f ORBITALS. At the fourth and higher levels, there are seven f orbitals in addition to the 4s, 4p, and 4d orbitals.
Construct the orbital diagram of the F^- ion. | Study.com Orbital diagram shows how electrons are distributed in various kinds of shells in the increasing order for a particular ion or element. The filling of electrons is studied and done by the help of ...
Electron Configuration for Fluorine (F) - UMD Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital.
Fluorine(F) electron configuration and orbital diagram Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund’s principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Shapes of Atomic Orbitals: Definition, Shapes of s, p, d, f The orbital wave function or Φ Φ is a mathematical function that is used to denote the coordinates of an electron. The square of Φ Φ indicates the probability of finding an electron in the orbital. Φ Φ helps in drawing out the boundary surface diagrams.
PDF Instructions Discussion Problem The F MO Diagram Instructions: (Part 2) Complete the F 2 orbital interaction diagram below. The 8 MOs should be labeled and ordered correctly relative to one another. Absolute position however is not expected. Draw dashed lines to show which atomic orbitals interact to form the MOs.
F Orbital Shape - EWT F Orbital. The sequence for the f block is unique. Beginning with lanthanum (Z=57) it starts a block that contains 15 elements. The 5 th level of a tetrahedron has 15 units. There are 15 elements for the f block (Z=57 to 71), although an odd number affects the number of orbitals (14 / 2 = 7). It converts a proton to neutron in the next d block to compensate, beginning with the 5d block.
Solved Construct the orbital diagram of the F- ion ... This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F- ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (24 ratings)



/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)












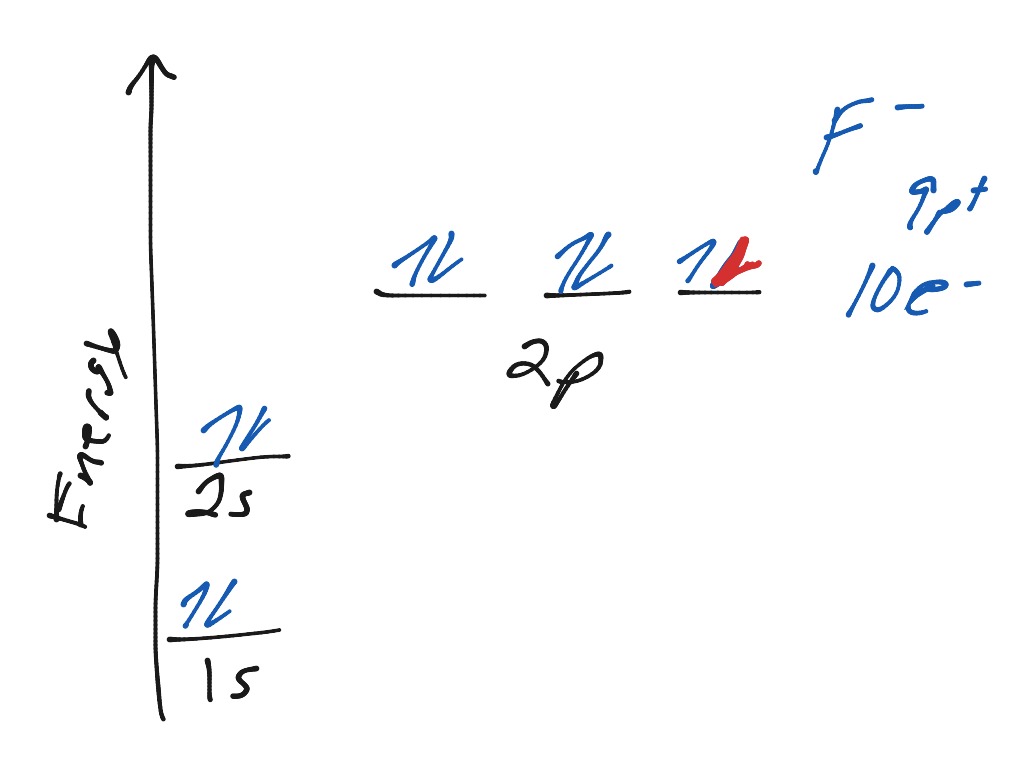








0 Response to "37 orbital diagram of f-"
Post a Comment