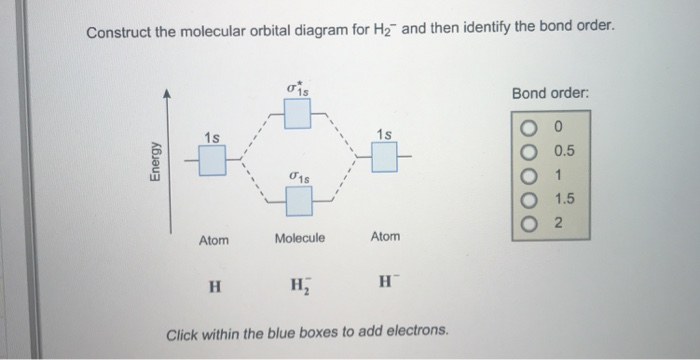
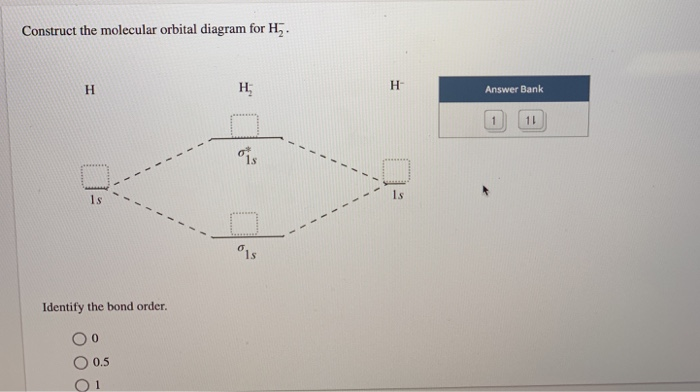
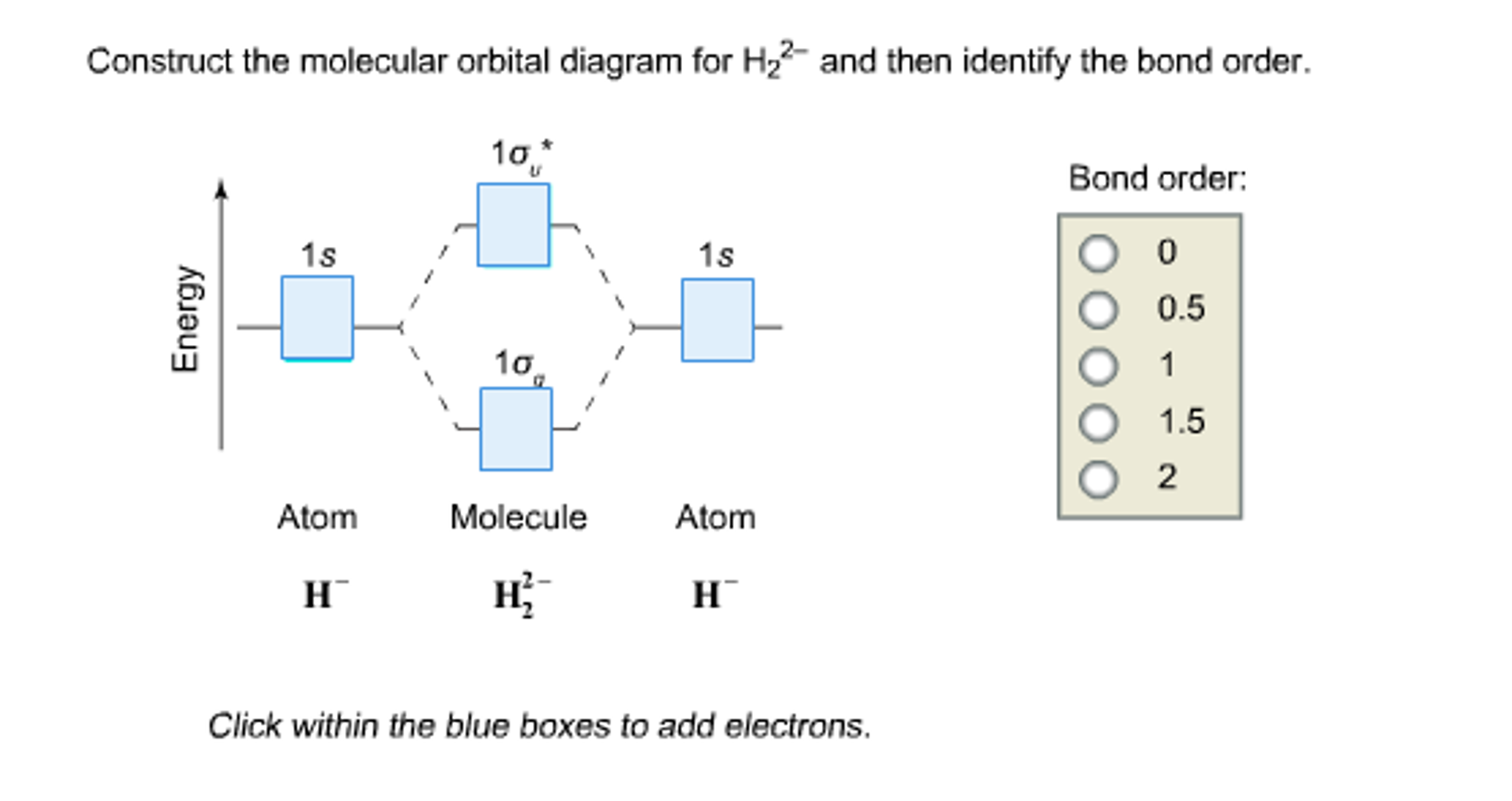
37 construct the molecular orbital diagram for h2– and then identify the bond order.
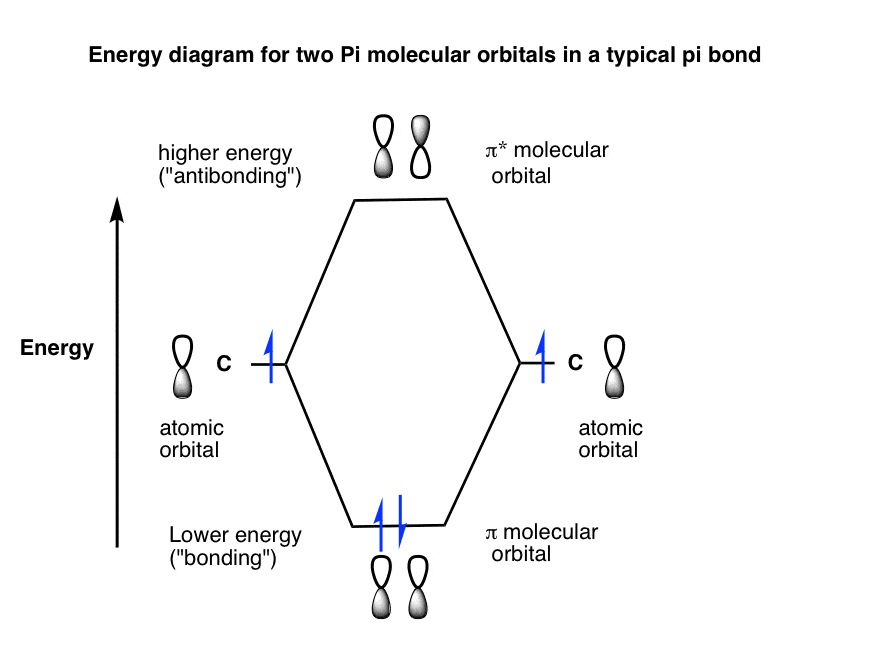
Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2. . , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. . −N a. . ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O. What is the bond order of H2-? - Quora Answer (1 of 4): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
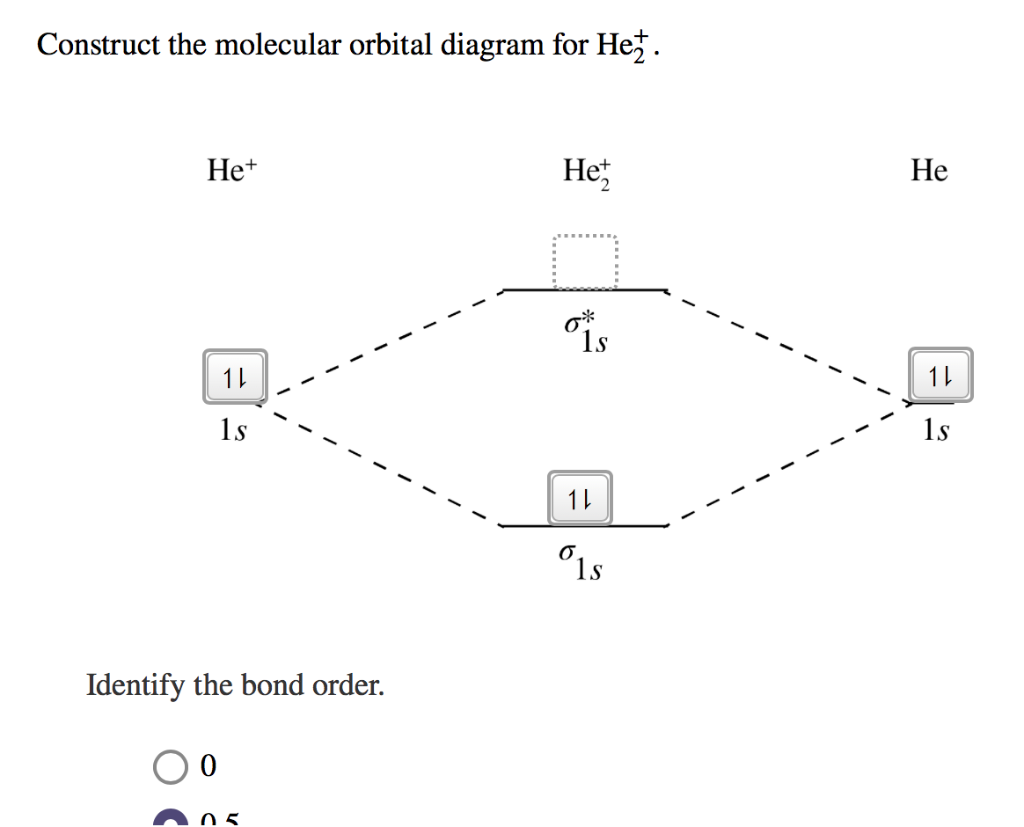
Construct The Molecular Orbital Diagram For He2 And Then ... Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. Since both molecular ions have a bond order of 1/2, they are approximately equally stable. Problem: Surprisingly, the hybridization of the starred oxygen in the following molecule is sp 2, not sp 3.

Construct the molecular orbital diagram for h2– and then identify the bond order.
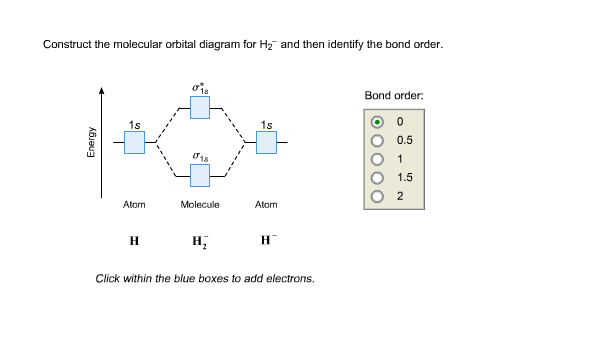
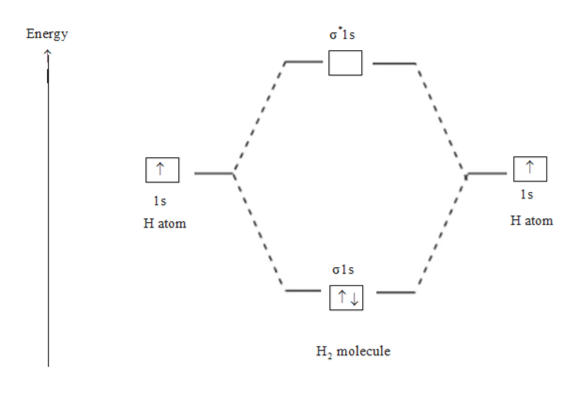
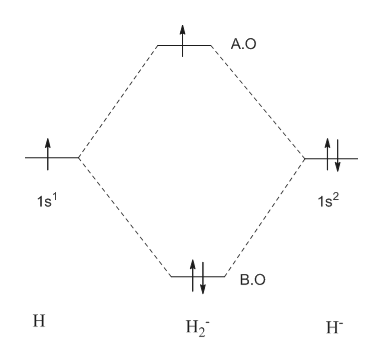
Construct the molecular orbital diagram for h2- and then ... ChemistryHelper2024 Since 1s shell of bonding orbital can accommodate only two electrons. So, next one electron will go into 1 s shell of anti-bonding orbital. - Bond order = (Bonding electrons - antibonding electrons) / 2 = (2 - 1) / 2 = 0.5 Advertisement New questions in Chemistry what is the pressure of 25.6g of cl2 occupying 15.6L at 28.6 c 5. Construct The Molecular Orbital Diagram For He2 And Then ... its molecular orbitals are constructed from the valence-shell orbitals of each bonding and antibonding combinations of each set are then formed, and from n level diagram that results is constructed by putting the molecular orbitals in order of if the z axis is identified with the internuclear axis, the 2s and 2pz orbitals on.show transcribed … Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
Construct the molecular orbital diagram for h2– and then identify the bond order.. OneClass: construct the molecular orbital diagram for He2 ... construct the molecular orbital diagram for He2+2 and Sapling Learning Map d mcanoe Construct the molecular orbital diagram for Hez and then identify the bond order Bond order: 1s 1s D0.5 Ï . 1s 0 1.5 Atom Molecule Atom HeHe He Click within the blue boxes to add electrons O Next, Exit- Show full question Answer + 20 Watch Molecular Orbital Diagram He2 - wiringall.com Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Even rather simple molecular orbital (MO) theory can be used to predict which from the bottom of the diagram because this is how MO diagrams are constructed , MOs are more antibonding than bonding MOs are bonding ... Construct the molecular orbital diagram for He22 and then ... Construct the molecular orbital diagram for He22 and then identify the bond order. Question 3 of 16 Map& Mapoob sapling leaning Construct the molecular orbital diagram for He and then identify the bond order. 1S Bond order O 0.5 O 1.5 1S 〠〠1s 1ー 1s 2 Atom Molecule Atom 2+ He He He Click within the blue boxes to add electrons. Construct the molecular orbital diagram for H2- and then ... Complete this molecular orbital diagram for CN- then determine the bond order. Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes.Bond order of CN-00.511.522.53. Construct the molecular orbital diagram for He. Answer Bank Energy Energy Atom He He He Identify...
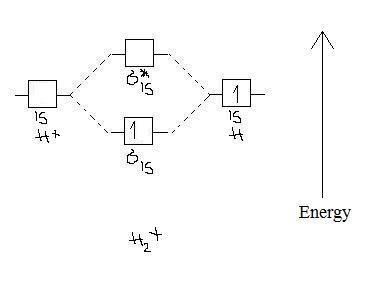
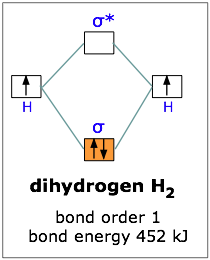
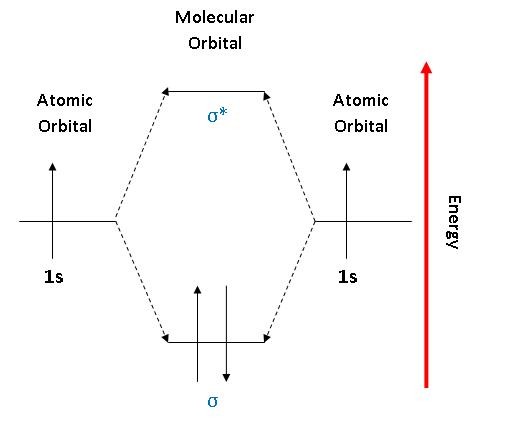
He2 2+ Molecular Orbital Diagram - Wiring Diagrams Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = 1/2 x ( Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Construct the molecular orbital diagram for He2 and then ... Construct the molecular orbital diagram for H2- and then identify the bond order. Construct the molecular orbital diagram for H2- and then identify the bond order.Bond order:00.511.52 Construct the molecular orbital diagram for N2 and then identify the bond order. What is the... How do I calculate the bond order for H2- and H2+? | Socratic Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. If you calculate their bond order, you get: BOH+ 2 = 1 2(Bonding − Antibonding) = 1 2 (1 − 0) = 1 2 BOH− 2 = 1 2(Bonding − Antibonding) = 1 2 (2 − 1) = 1 2 Construct the molecular orbital diagram for H2 and then ... Construct the molecular orbital diagram for H2 and then identify the bond order. Click within the blue boxes to add electrons. A solution of H2SO4(aq) with a molal concentration of 2.24 m has a density of 1.135 g/mL. What is the molar concentration... Posted one month ago View Answer Q:
OneClass: Construct the molecular orbital diagram for H2 ... 1 Nov 2019 Construct the molecular orbital diagram for H2 and then identify the bond order. Make sure you add electrons to the boxes corresponding to the MOs for the molecule and to the boxes corresponding to the AOs for the two atomic species. Bond order: 1s o 0.5 O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons. Construct The Molecular Orbital Diagram For H2 And Then ... Indicate the lowest energy electron excitation in this ion by identifying the initial and.Show transcribed image text Construct the molecular orbital diagram for H2 and then identify the bond order. Click within the blue boxes to add electrons. Click within the blue boxes to add electrons% (15). Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Construct The Molecular Orbital Diagram For He2 And Then ... its molecular orbitals are constructed from the valence-shell orbitals of each bonding and antibonding combinations of each set are then formed, and from n level diagram that results is constructed by putting the molecular orbitals in order of if the z axis is identified with the internuclear axis, the 2s and 2pz orbitals on.show transcribed …
Construct the molecular orbital diagram for h2- and then ... ChemistryHelper2024 Since 1s shell of bonding orbital can accommodate only two electrons. So, next one electron will go into 1 s shell of anti-bonding orbital. - Bond order = (Bonding electrons - antibonding electrons) / 2 = (2 - 1) / 2 = 0.5 Advertisement New questions in Chemistry what is the pressure of 25.6g of cl2 occupying 15.6L at 28.6 c 5.










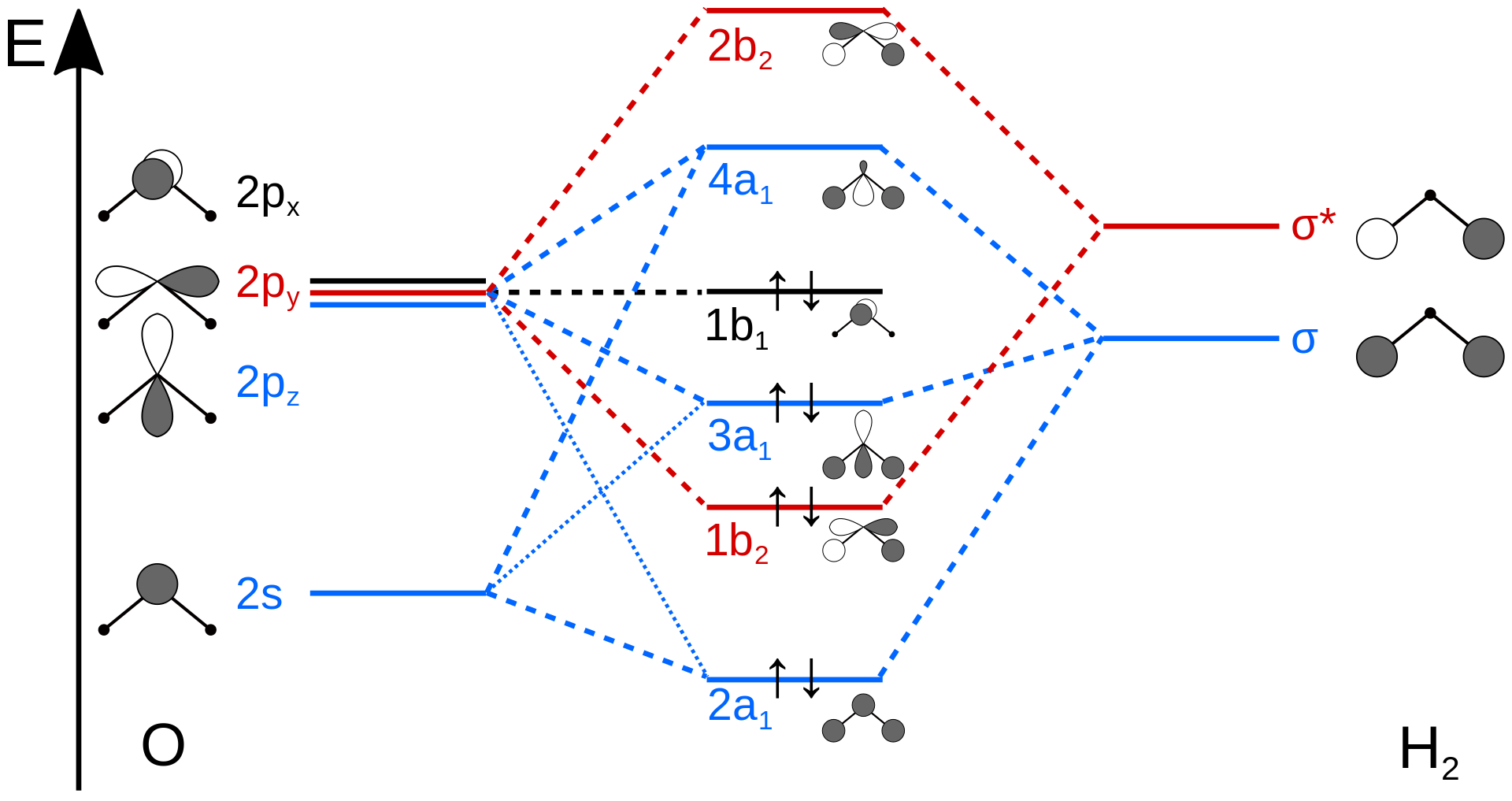

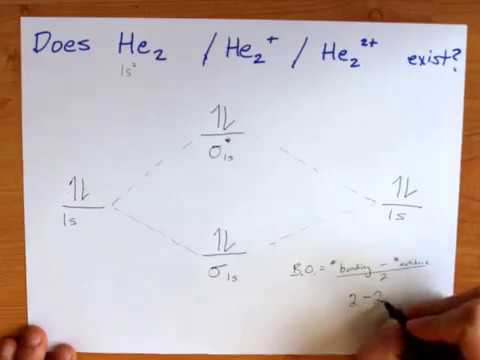

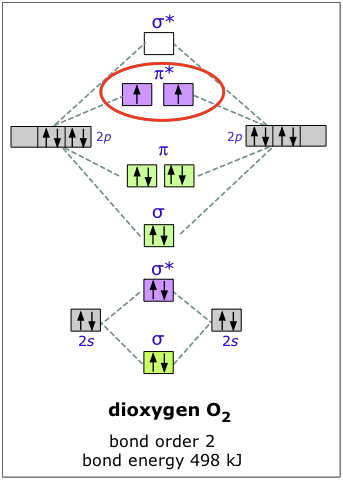

0 Response to "37 construct the molecular orbital diagram for h2– and then identify the bond order."
Post a Comment