39 orbital diagram for beryllium
7. nov. 2021 ... From the orbital diagram, we can write the electron configuration in an ... The next element is beryllium, with Z = 4 and four electrons. November 23, 2015 - Get an answer for 'Write each element's orbital notation and complete electron configuration -beryllium - aluminum -nitrogen -sodium' and find homework help for other Science questions at eNotes
In this video we will write the electron configuration for Be 2+, the Beryllium ion. We’ll also look at why Beryllium forms a 2+ ion and how the electron con...

Orbital diagram for beryllium
The molecular orbital diagram of the BeCl2 molecule is drawn by the combination of Beryllium atomic orbitals and chlorine group orbitals. As there are two chlorine atoms and hence, first they combine to form group orbitals. The electronic configuration of Cl is [Ne] 3s23p5. Complete answer: Diagrams representing the arrangement of orbitals in increasing order of their energy levels are known as orbital energy diagrams or energy ... (i) Be2 molecule: The electronic configuration of Be(Z = 4) is:4 Be 1s2 2s1Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
Orbital diagram for beryllium. Well, the atomic orbital (AO) ordering is quite normal and predictable. BERYLLIUM AO ENERGY ORDERING "Be"'s ground-state electron configuration is the one you've probably learned by now, which is 1s^2 2s^2; that indicates that the 2s is higher in energy (but you knew that), so all you really have is a 1s AO, and then a 2s AO substantially higher in energy. That's the AO diagram. So, literally ... molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ... The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (or ). Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. Mar 21, 2019 · Another way to identify the location of each electron is via an orbital diagram. To designate the The orbital diagram for beryllium is shown here. The electron. Jul 12, Electron configurations and orbital diagrams can be determined by applying the An atom of the alkaline earth metal beryllium, with an atomic. Well, the atomic orbital (AO) ordering is quite normal and predictable. BERYLLIUM AO ENERGY ORDERING. To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. Beryllium Elec Config 1s 2 2s 2 Orbital Diagram 2s 1s Beryllium is alloyed with from CHEM 160 at University of Nebraska, Kearney
Electronic configuration of the Beryllium atom. Valence electrons. Orbital diagram. Find step-by-step Chemistry solutions and your answer to the following textbook question: Write each element's orbital notation and complete electron configuration. A. beryllium B. aluminum C. nitrogen D. sodium. The beryllium atom, with only four electrons, ... Draw "orbital box" diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. Show how hybrid orbitals are involved in the molecules methane, water, and ammonia. ... July 19, 2021 - The next element after beryllium is boron. Since the 2s orbital is completely filled, a new type of orbital must be used for the fifth electron. There are three 2p orbitals available, and any of them might be used. Plate 5 shows the fifth electron (color-coded purple) occupying the 2px orbital.
As one moves across the second period, electrons are successively added. With beryllium (Z = 4), the 2s sublevel is complete and the 2p sublevel begins with boron (Z = 5). Since there are three 2 p orbitals and each orbital holds two electrons, the 2p sublevel is filled after six elements.
Answer to A molecular orbital diagram for beryllium (Be) is shown. Will beryllium exist as a molecule according to molecular orbit...
The Bohr Model of Beryllium(Be) has a nucleus that contains 5 neutrons and 4 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Beryllium contains 2 electrons that also called valence electrons.
Beryllium has a configuration of 1s22s2. Boron Through Neon - The 2p Orbitals Now that the 1s and 2s orbitals are filled, the next lowest energy orbitals are the three 2p orbitals. Boron has a configuration of 1s22s22p1. Beginning with carbon, we start to see Hund's rule come into play.
Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s 2 2s 2.
Comprehensive data on the chemical element Beryllium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Beryllium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their ...
in this problem, we are asked to draw the molecular orbital diagram for the dia tom between beryllium and carbon. So ultimately what we're first going to do is separate it into two states beryllium and carbon and we'll write the electron configuration for each. So beryllium is one S 22 S two and carbon is one S 22 S two to P. Two. So for molecular orbital's would just draw the atomic orbital's ...
Chem4Kids.com! Beryllium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
Electrons & Oxidation ; Oxidation States, 2 ; Electrons Per Shell, 2 2 ; Electron Configuration, [He] 2s ...
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Apr 16, 2019 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining .. Beryllium has an electron configuration 1s22s2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in .Feb 23, · A quiz solution for Inorganic Chemistry in which students were prompted to draw the molecular orbital diagram for beryllium hydride.
The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus.
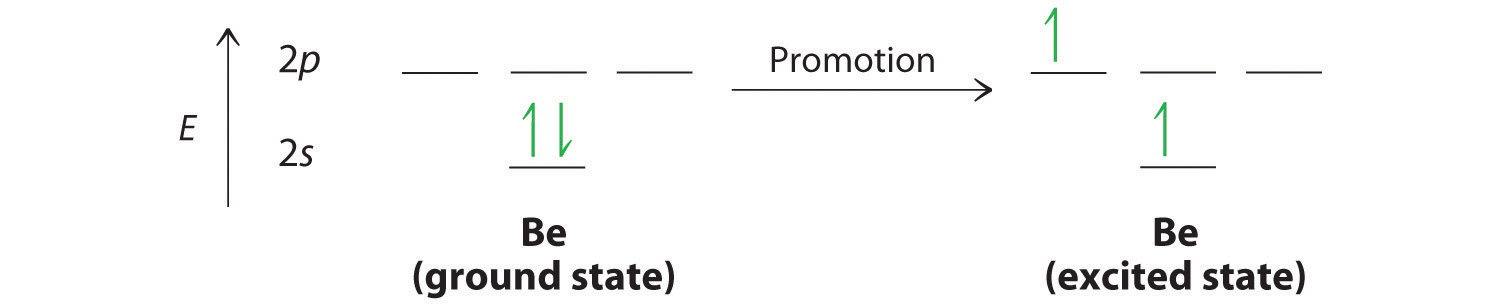
First of all write orbital diagrams for Beryllium and Hydrogen. Beryllium has 4 electrons and Hydrogen has 1 electron. Before hybridization, Beryllium does have unpaired electrons to form bonding.So,one electron from 2s orbital jumps fron 2s level to 2p level and the orbitals hybtidize to form hybrid orbitals.
Now, the 2s orbital of the beryllium atom fuses with its 2p orbital and hence, form two sp hybrid orbitals of equivalent energy, which align themselves in a linear geometry. The sp hybrid orbital of the beryllium atom overlap with the 1s atomic orbital of the hydrogen atom, which is shown in the orbital diagram of the beryllium hydride molecule ...
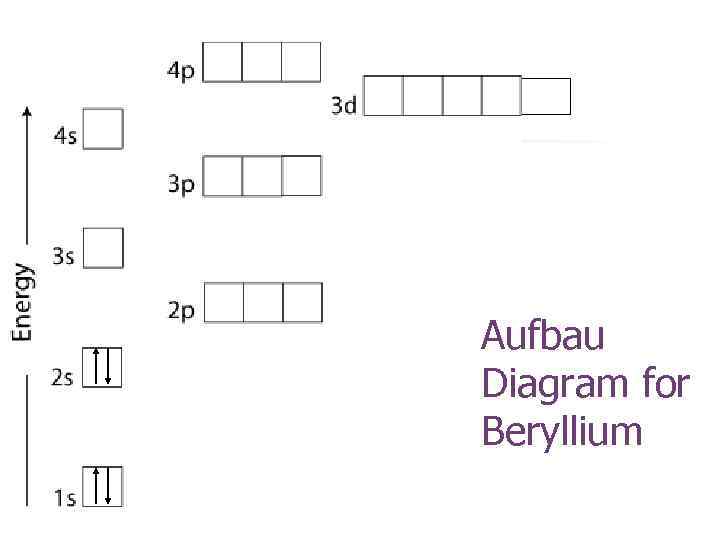
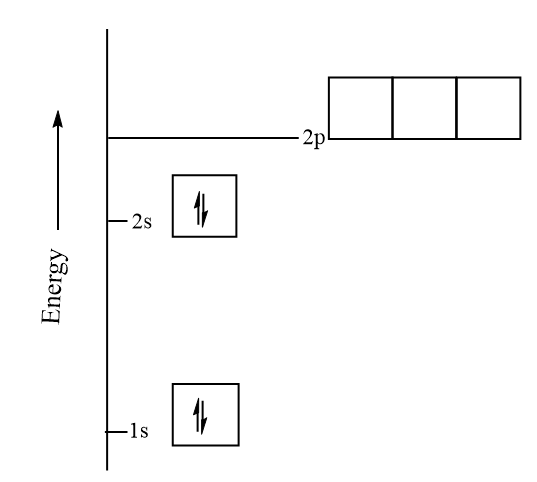
The orbital diagram for beryllium is shown here. The electron configuration is 1s 2 2s 2. The fourth electron is placed in the 2s orbital. The energy required to pair the first 2s electron is less than the energy required to place the electron into the 2p orbital. The next element is boron with 5 electrons.
Scientist Niels Bohr was the first to give an idea of the atom orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there.The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit.These orbits are expressed by n. [ n = 1,2 3 4 . . .] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. [Where, n = 1,2 3,4. . .] Now, n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 32= 32 electrons. The atomic number is the number of electrons in that element. The atomic number of beryllium is 4. That is, the number of...
A molecular orbital diagram for beryllium (Be) is shown. Will beryllium exist as a molecule according to molecular orbital theory? Yes: No: Because: a. Electrons in bonding orbitals make the species more stable and electrons in antibonding orbitals make the species less stable. b.
Molecular Orbital Diagram for Beryllium Dimer (Be2) Fill from the bottom up, with 4 electrons total. Bonding Order is 0, meaning it does not bond, and it is ...
To write the orbital diagram for the Beryllium atom (Be) first we need to write the electron configuration for just Be. To do that we need to find the numbe...
February 6, 2021 - As the electrons located in the outermost orbit is considered as valence electrons and beryllium has 2 electrons in its outer shell. Filed Under: Period Table Tagged With: Beryllium Electron Configuration, Electron Configuration For Be, Electron Configuration For Beryllium, How Many Valence ...
February 22, 2018 - Answer (1 of 8): Well, as the first point it is to be noted that an atom forms a molecule in order to get stabilised. In other word we can say that in order to form a molecule the energies of the atomic orbitals should be lowered in the molecule. Now, we know that in a molecule the atomic orbita...
Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s 2 2s 2.
Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s 2 2s 2.
A step-by-step description of how to write the electron configuration for Beryllium (Be). In order to write the Be electron configuration we first need to k...
Jan 21, 2019 · Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of beryllium-9 (atomic number: 4), the most common. Since the 2s orbital is completely filled, a new type of orbital must be of one more atom, carbon, with the aid of the color-coded diagrams. Beryllium is the fourth element with a total of 4 electrons.
Looking at the orbital diagram for the valence shell of Beryllium in BeCl 2, it shows a pair of electrons in the 2s subshell. However, in order for Be to form two covalent bonds (see Lewis Structure, below), it clearly must have two single electrons in each of two orbitals. We theorize
Welcome to Science!
Draw the molecular orbital diagram for: (i) Be2 (ii) B2 and predict bond order and magnetic properties. (i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s2 2s1 Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
A quiz solution for Inorganic Chemistry in which students were prompted to draw the molecular orbital diagram for beryllium hydride.
Nov 30, 2018 · This gives us an orbital filling diagram of. The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here. The electron configuration is 1s 2 2s 2. The fourth electron is placed in the 2s orbital. The energy required to pair the first 2s electron is less than the energy required to place the electron into the 2p orbital. Our beryllium page has over facts that span different quantities.
Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2 s orbital. An atom of boron (atomic number 5) contains five electrons.
The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here. The electron configuration is 1s22s2. The fourth electron is placed in the 2s orbital. The energy required to pair the first 2s electron is less than the energy required to place the electron ...
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is:4 Be 1s2 2s1Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
Complete answer: Diagrams representing the arrangement of orbitals in increasing order of their energy levels are known as orbital energy diagrams or energy ...
The molecular orbital diagram of the BeCl2 molecule is drawn by the combination of Beryllium atomic orbitals and chlorine group orbitals. As there are two chlorine atoms and hence, first they combine to form group orbitals. The electronic configuration of Cl is [Ne] 3s23p5.












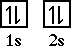







:max_bytes(150000):strip_icc()/berylliumatom-58b6028f3df78cdcd83d8f11.jpg)
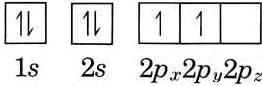
0 Response to "39 orbital diagram for beryllium"
Post a Comment