40 lewis dot diagram for nitrogen
What is the Lewis dot structure of N2O4? Dinitrogen tetroxide is a one of the oxide of nitrogen and are two nitrogen atoms are located at center of the molecule. In Lewis Structure of N2O4, two oxygen atoms have connected to one nitrogen atom. There are charges on atoms in Lewis Structure of N2O4. What is the molecular shape of N2O4?
A Lewis structure for NO would look like: Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom. The double bar between the two chemical symbols (=) means that nitrogen and oxygen share a double bond—2 pairs of electrons. Lastly, there is a single unpaired electron on the nitrogen atom.
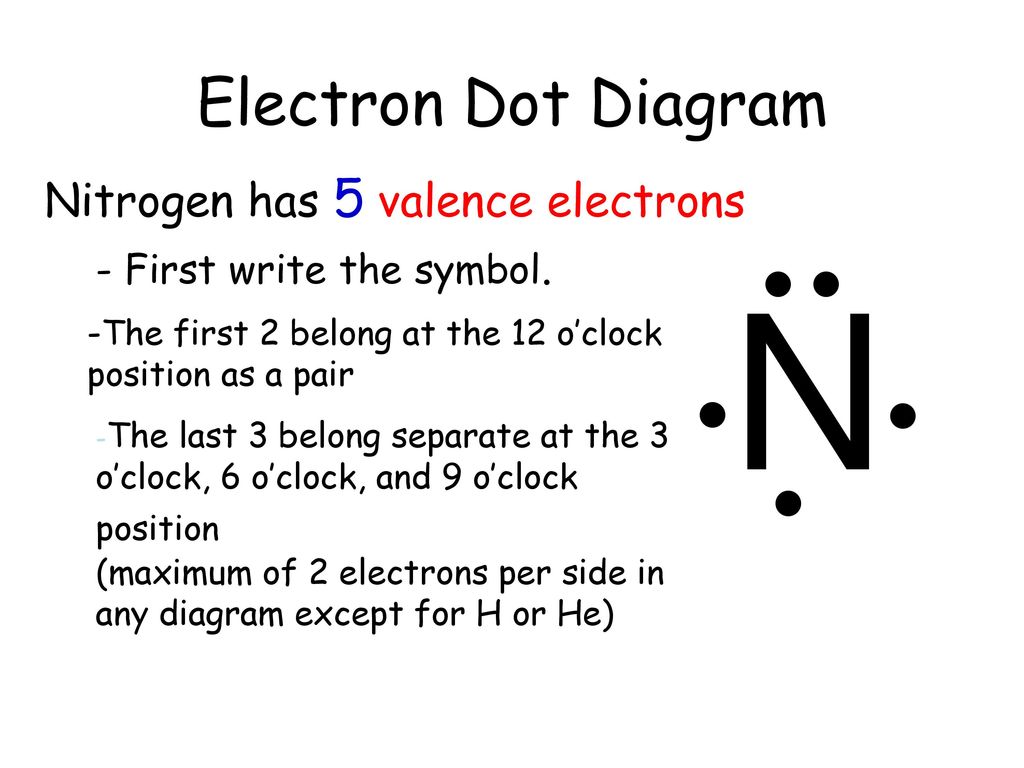
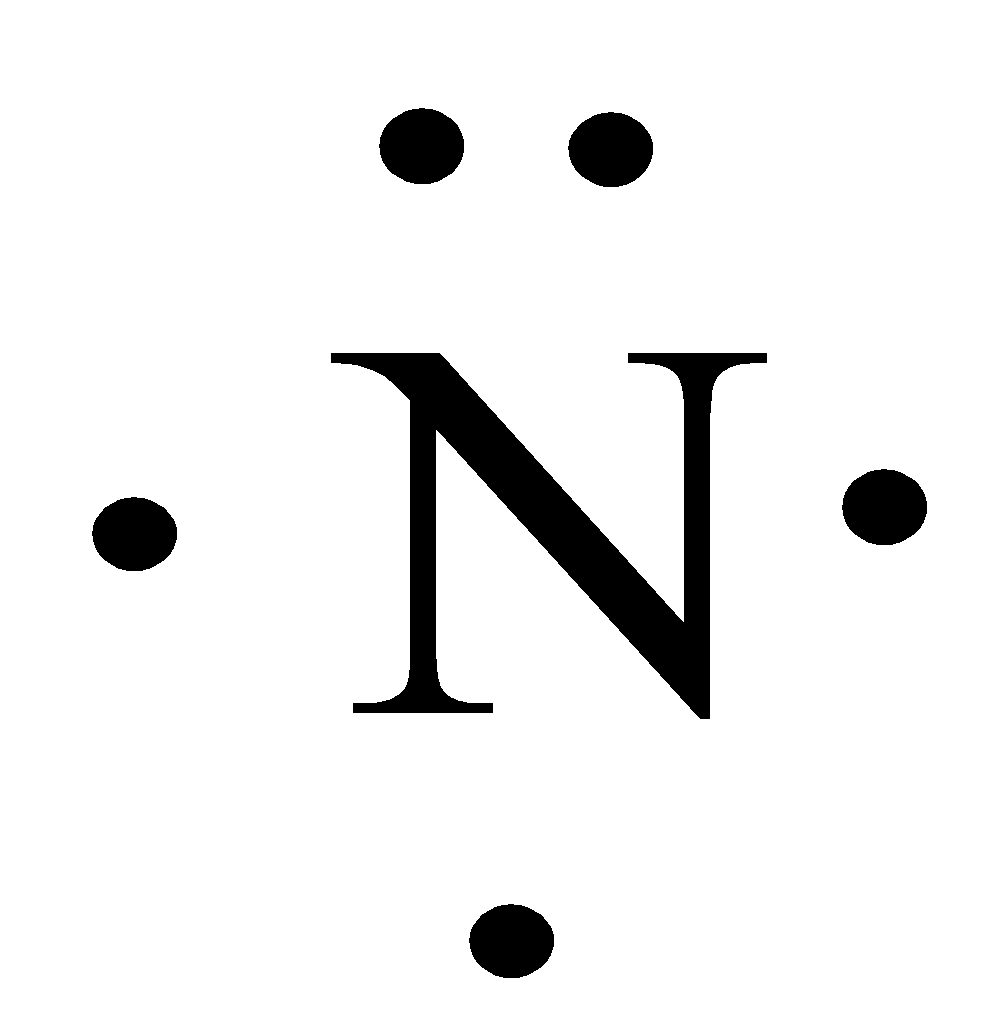
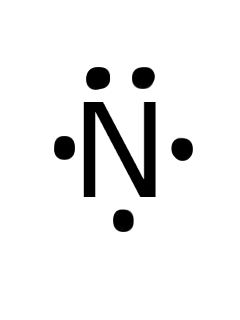
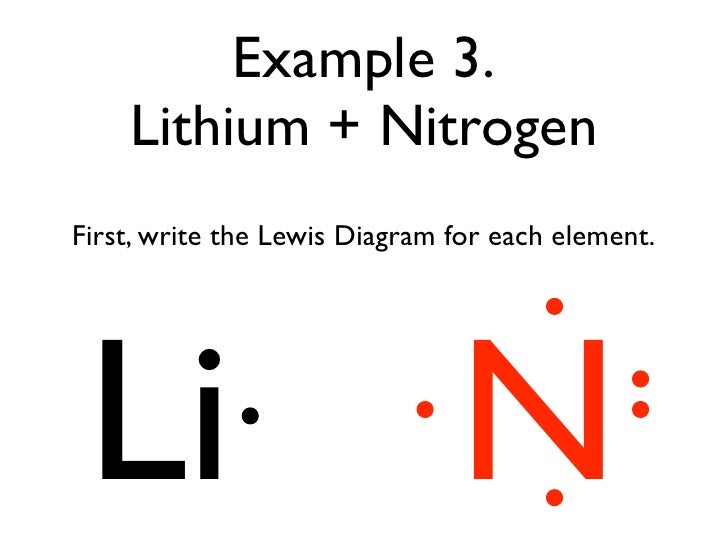
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . ....

Lewis dot diagram for nitrogen
what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
Draw the electron dot structure of Nitrogen molecule [N=7] · Solution · Nitrogen molecule. N=7⇒2K5L N=7⇒2K5L Nitrogen atom shares three electrons forming ...1 answer · Top answer: Nitrogen molecule N = 7 & 2 & 5 & K & L N = 7 & 2 & 5 & K & L Nitrogen atom shares three electrons forming a triple covalent bond.
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Lewis dot diagram for nitrogen.
Lewis dot diagram for nitrogen. Lewis structure electron dot diagram for ammonia or note that there are 3 covalent bonds 3 bonding pairs of electrons in total and that there is a lone pair non bonding pair of electrons on the nitrogen atom. It is the lightest pnictogen and at room temperature it is a transparent odorless diatomic gas.
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
Apr 11, 2016 — The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of ...1 answer · Explanation: The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration ...
In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs .In N2 Lewis structure,there are ten ...3 answers · 4 votes: As nitrogen is in fifth group in periodic table therefore it will have five electrons in the ...
on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use .
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.Each fluorine atom has three lone pairs. Steps of drawing lewis structure of NF 3. You have to follow few steps to draw the lewis structure of NF 3.Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps ...
Lewis Dot Diagram for Nitrogen. what is lewis dot diagram of nitrogen gas answers the lewis dot structure of a nitrogen atom would be the capitol letter n with the five valence electrons represented by two dots above it one to the lewis dot structure for nitrogen atom n a step by step explanation of how to draw the lewis dot structure for n nitrogen i show you where nitrogen is on the periodic ...
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
Get all of Hollywood.com's best Movies lists, news, and more.
Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. Single bonds are represented by a pair of dots or one line between atoms. Likewise, what is the electron dot diagram for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-).
Nitrogen trichloride (NCl3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar.
Answer (1 of 2): NO3- is the nitrate ion, and the conjugate base of nitric acid.Two of the three oxygens bound to the nitrogen atom have their three outer lone pairs, with a sigma bond to the nitrogen. One of the oxygens is the exception and has only two lone pairs a since it has a double bond co...
Ammonia is NH3. Nitrogen has five valence electrons and each hydrogen brings one to the molecule. It's easy to see that those three electrons from the hydrogens ...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for ... nitrogen, 1 s 2 2 s 2 2 p 3, 5 valence electrons.Nitrogen: 1 s 2 2 s 2 2 p 3Neon: 1 s 2 2 s 2 2 p 6Lithium: 1 s 2 2 s 1Beryllium: 1 s 2 2 s 2
Lewis Dot Structures. from. Chapter 20 / Lesson 6. 20K. Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn ...
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ...
Examples Of Lewis Electron Dot Structures. Lewis Electron Dot Structure for O 2 molecule. An oxygen atom has 6 valence electrons in the valence shell and so it needs 2 more to complete the octet. So both the atoms contribute two atoms each for the bond. Hence a double bond is formed. Lewis Electron Dot Structure for the molecule: CO 2
For diatomic nitrogen, the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms: This triple bond is very strong. The strength of the triple bond makes the N 2 molecule very stable against chemical change, and, in fact, N 2 is considered to be a chemically inert gas .There is a relationship between the ...
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […]
A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we ...Oct 25, 2016 · Uploaded by Wayne Breslyn
So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX3N1 So, according to the VSEPR chart, if the molecule has the formula of AX3N1, it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral.
The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
Note: The most important thing about the Lewis dot structure is that only valence electrons take part in chemical bonding. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2.


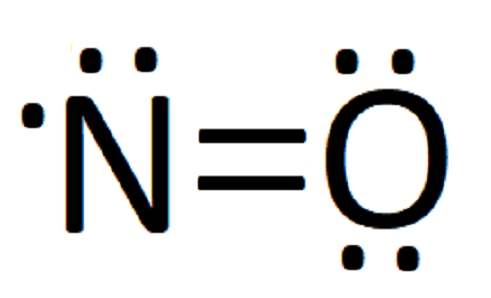



![Expert Answer] electron dot structure of Nitrogen molecule ...](https://hi-static.z-dn.net/files/d68/c596d1dd5842287e6938ce11c21acb41.png)






![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)

















0 Response to "40 lewis dot diagram for nitrogen"
Post a Comment