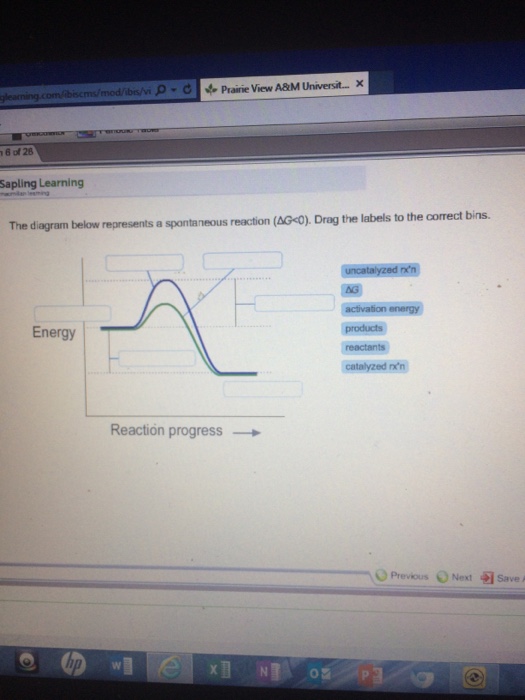
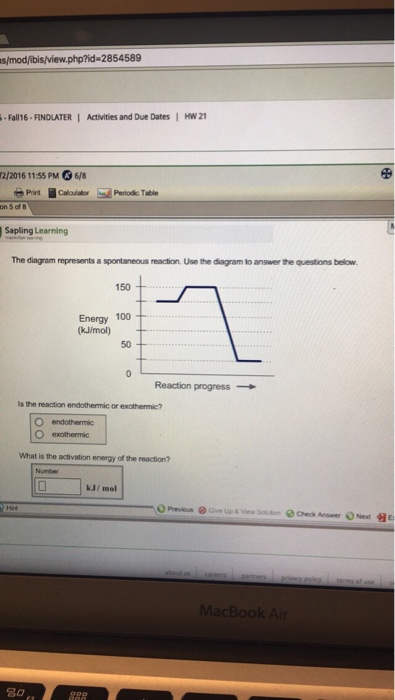
36 the diagram below represents a spontaneous reaction (δg
Question: The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree ... The diagram below represents a spontaneous reaction (deltaG degree. ... Calculate the standard change in Gibbs free energy for the following reaction at 25 ...
The Gibbs free energy change (ΔG) and how it's related to reaction ... starting and final states (the purple lines on the diagrams below), while spontaneity ...
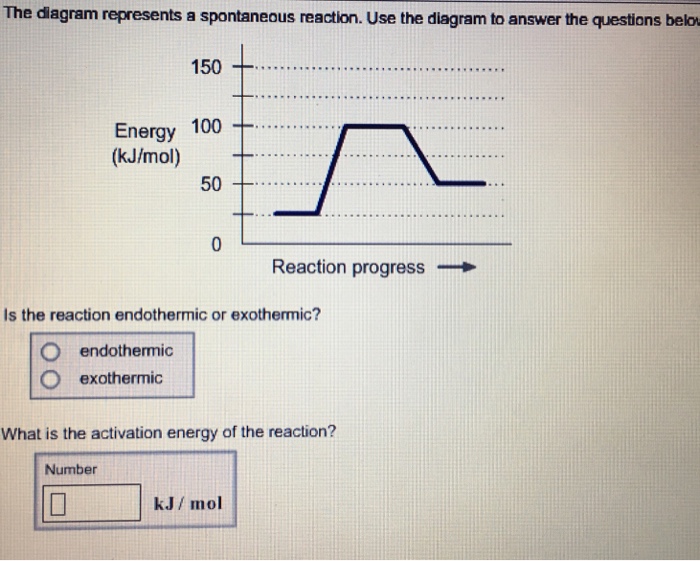
The diagram below represents a spontaneous reaction (δg
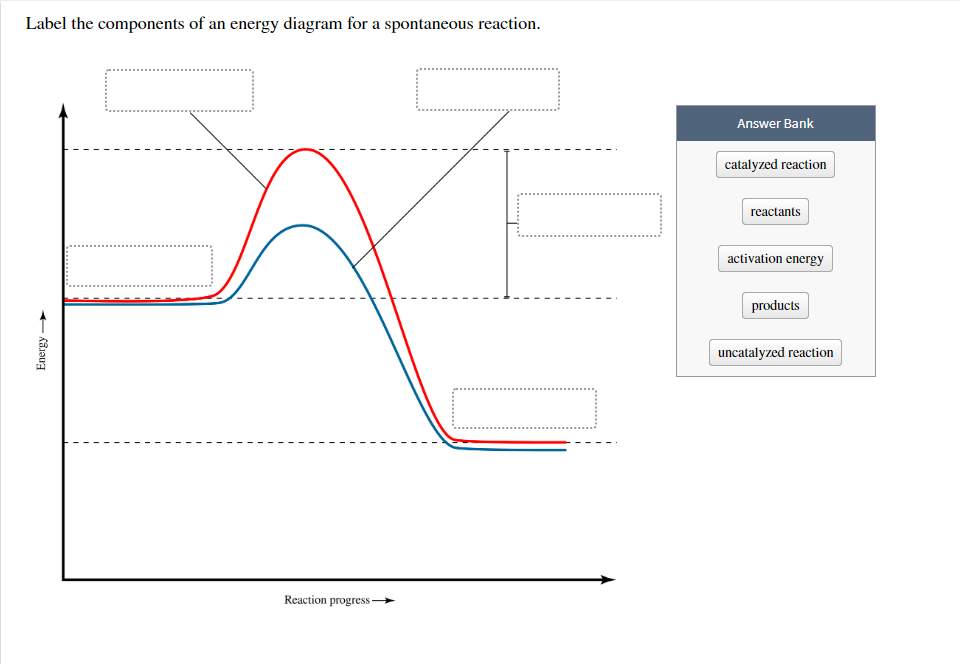
(a) The reaction glyceraldehyde 3-phosphate 1,3-bisphosphoglycerate should be inhibited when levels of NADH fall. (b) The ΔG° for the oxidation of the aldehyde group on glyceraldehyde 3-phosphate to form a carboxylic acid is more negative than the ΔG° for ATP hydrolysis. Problem: The diagram below represents a spontaneous reaction (deltaG degree ... Energy change (ΔH or ΔG˚): is the difference in energy between products and ...1 answer · Top answer: We’re being asked to label the given energy diagram.Recall that an energy diagram is usually read from left to right. The components of energy ... Apr 13, 2021 · which represents the transport of cupric ion from a region of higher concentration to one of lower concentration. The driving force for this process is the free energy change ΔG associated with the concentration gradient (C 2 – C 1), sometimes known as the free energy of dilution: \[ΔG_{dilution} = RT \ln(C_2 – C_1)\]
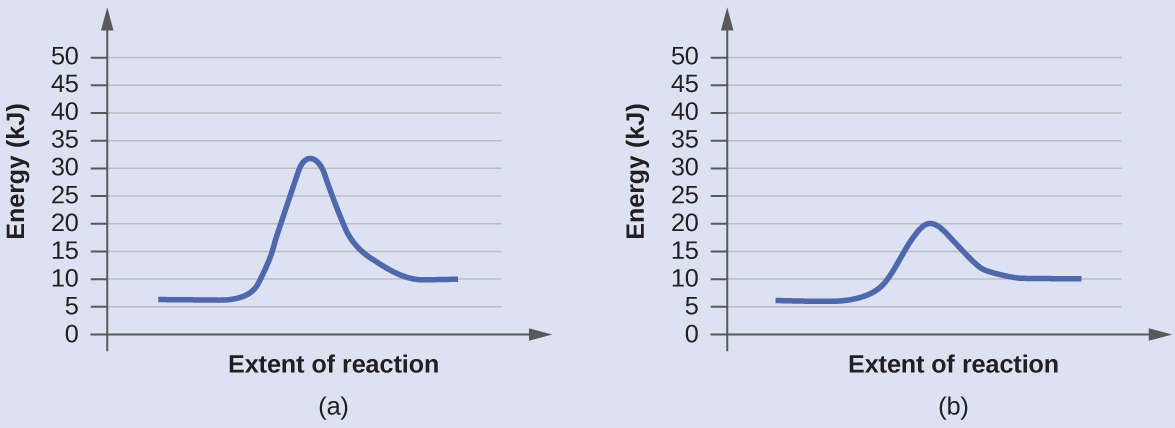
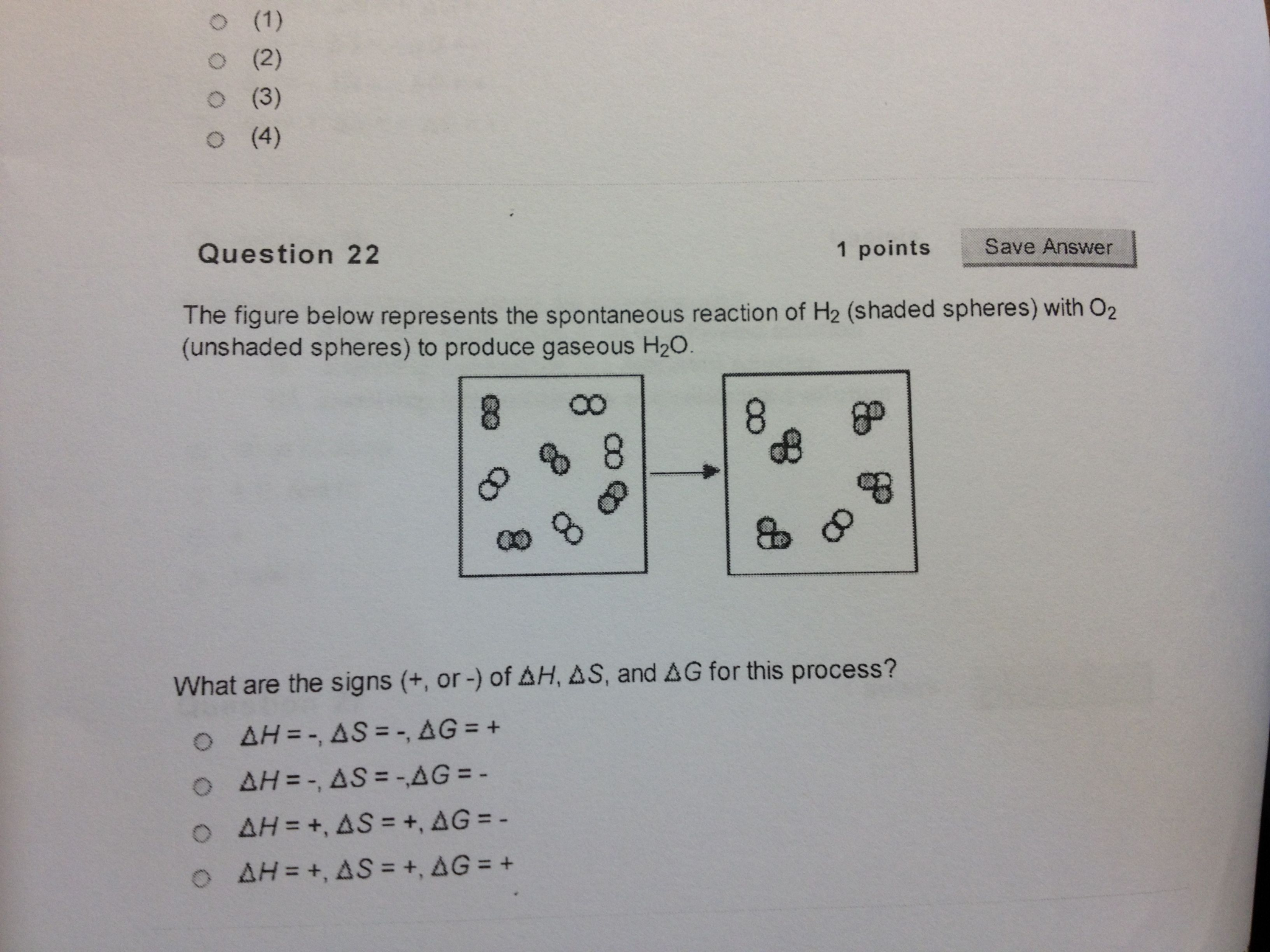
The diagram below represents a spontaneous reaction (δg. Aug 07, 2021 · For a spontaneous reaction, E cell is positive and ΔG (Gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Thus, when ΔG is negative the reaction is spontaneous. Merging electrochemistry with thermodynamics gives this formula: \[\Delta G = -n F E_{cell} \] The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether ΔH, ΔS, and ΔG for this reaction are positive, negative, or zero. Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation. At its equilibrium point (point 3), there are more products than reactants. If the reaction starts at a composition to the right of point 3 on the diagram, the composition will tend to move to the left. If the initial composition is to the left of point 3 on diagram, the reaction will tend to proceed to the right. Exergonic reactions release free energy; the ∆G for that reaction will be a ... of rust above, the breakdown of sugar involves spontaneous reactions, ...
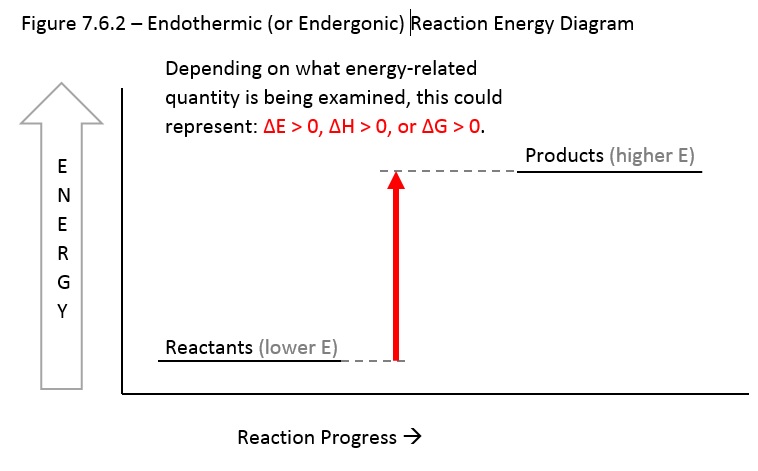
Figure 1.18 The Change in the Gibbs free energy (ΔG) is the Driving Force of the Reaction. In this diagram, equilibrium (where ΔG = 0) is represented by the black line of the horizontal axis. Using Le Chatlier’s Principle, it is possible to predict the behavior of a reaction when perturbations are made that shift the reaction out of ... 1) ΔG. 3) ΔS. 2) ΔH. 4) ΔT. 13. Which phase change represents a decrease in entropy? ... The potential energy diagram below represents a reaction. If the ΔG is negative the reaction is spontaneous, and if the ΔG is positive the reaction is not spontaneous. If ΔG = 0 the reaction is in a state of equilibrium, meaning that the forward reaction is happening at an equal rate as the reverse reaction and there is no net gain or loss of reactants and products. In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction.1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ...
Apr 13, 2021 · which represents the transport of cupric ion from a region of higher concentration to one of lower concentration. The driving force for this process is the free energy change ΔG associated with the concentration gradient (C 2 – C 1), sometimes known as the free energy of dilution: \[ΔG_{dilution} = RT \ln(C_2 – C_1)\] Problem: The diagram below represents a spontaneous reaction (deltaG degree ... Energy change (ΔH or ΔG˚): is the difference in energy between products and ...1 answer · Top answer: We’re being asked to label the given energy diagram.Recall that an energy diagram is usually read from left to right. The components of energy ... (a) The reaction glyceraldehyde 3-phosphate 1,3-bisphosphoglycerate should be inhibited when levels of NADH fall. (b) The ΔG° for the oxidation of the aldehyde group on glyceraldehyde 3-phosphate to form a carboxylic acid is more negative than the ΔG° for ATP hydrolysis.
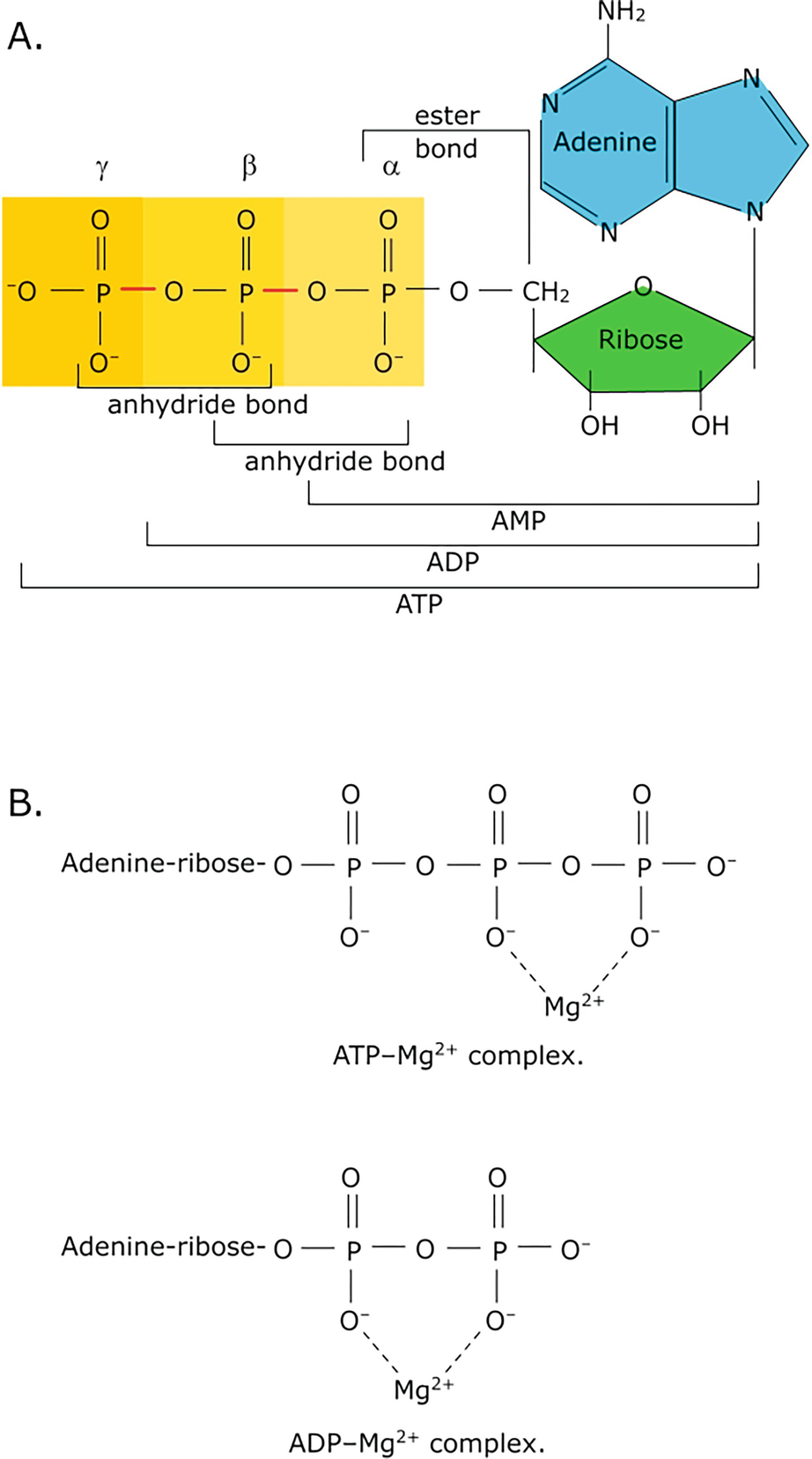
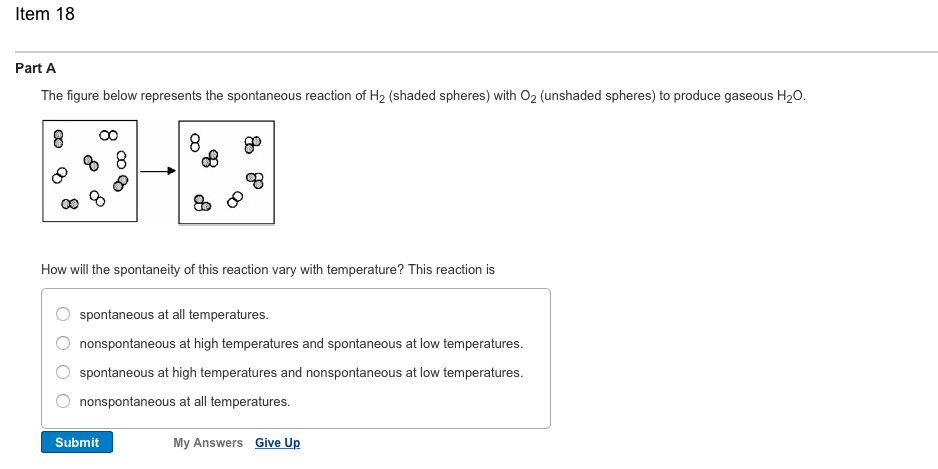
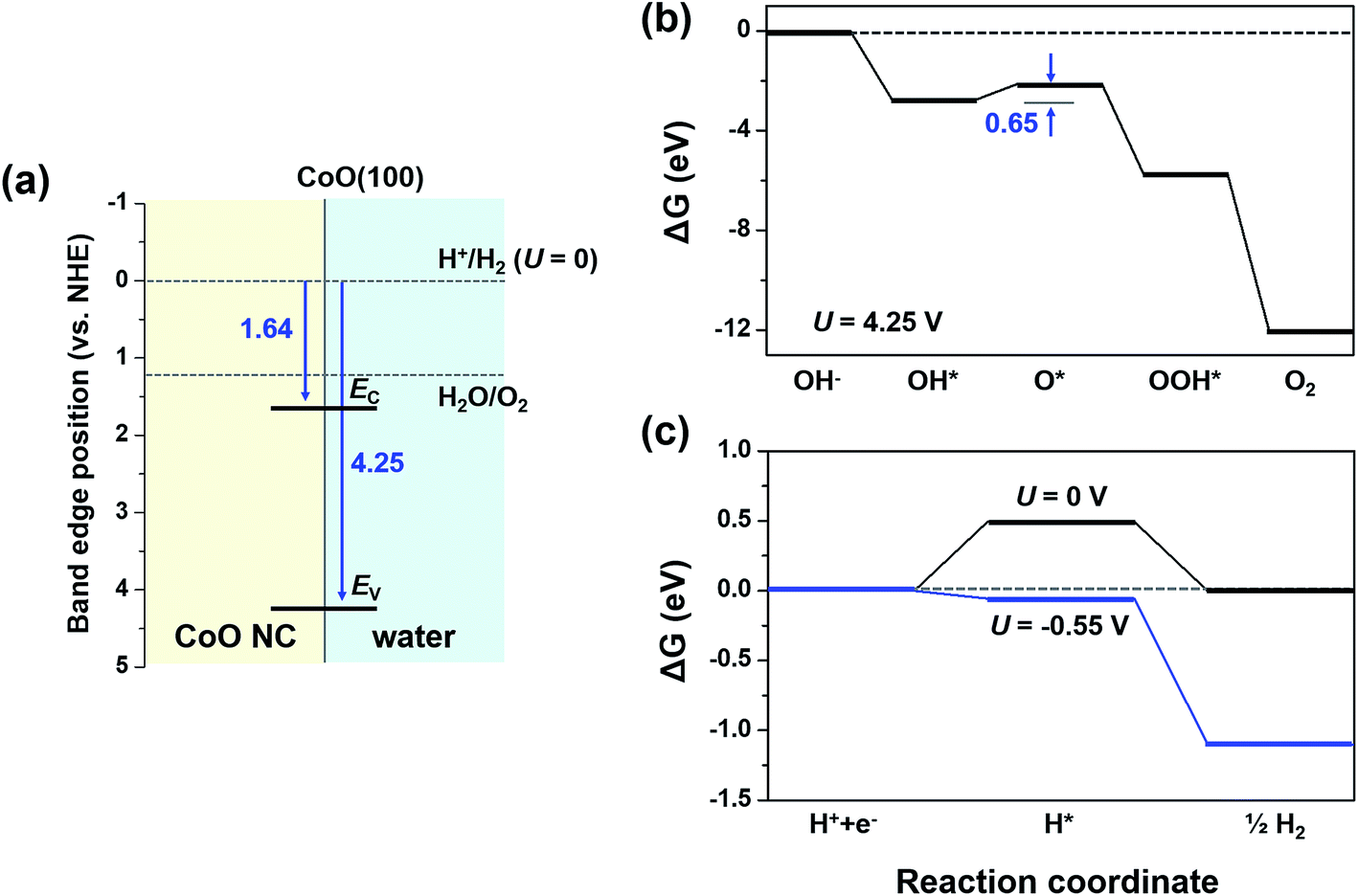
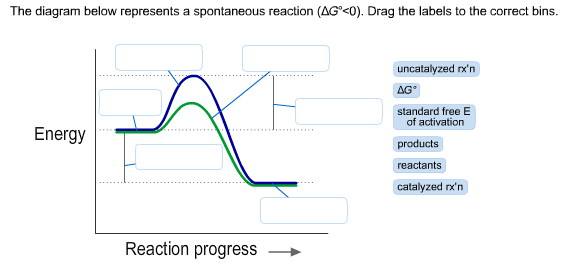



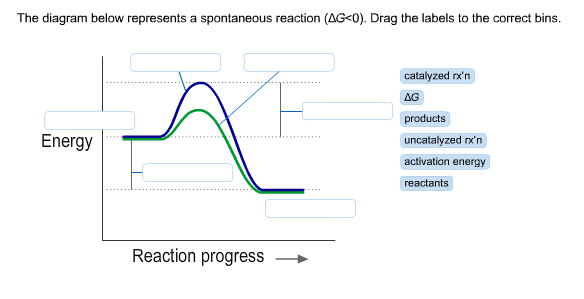



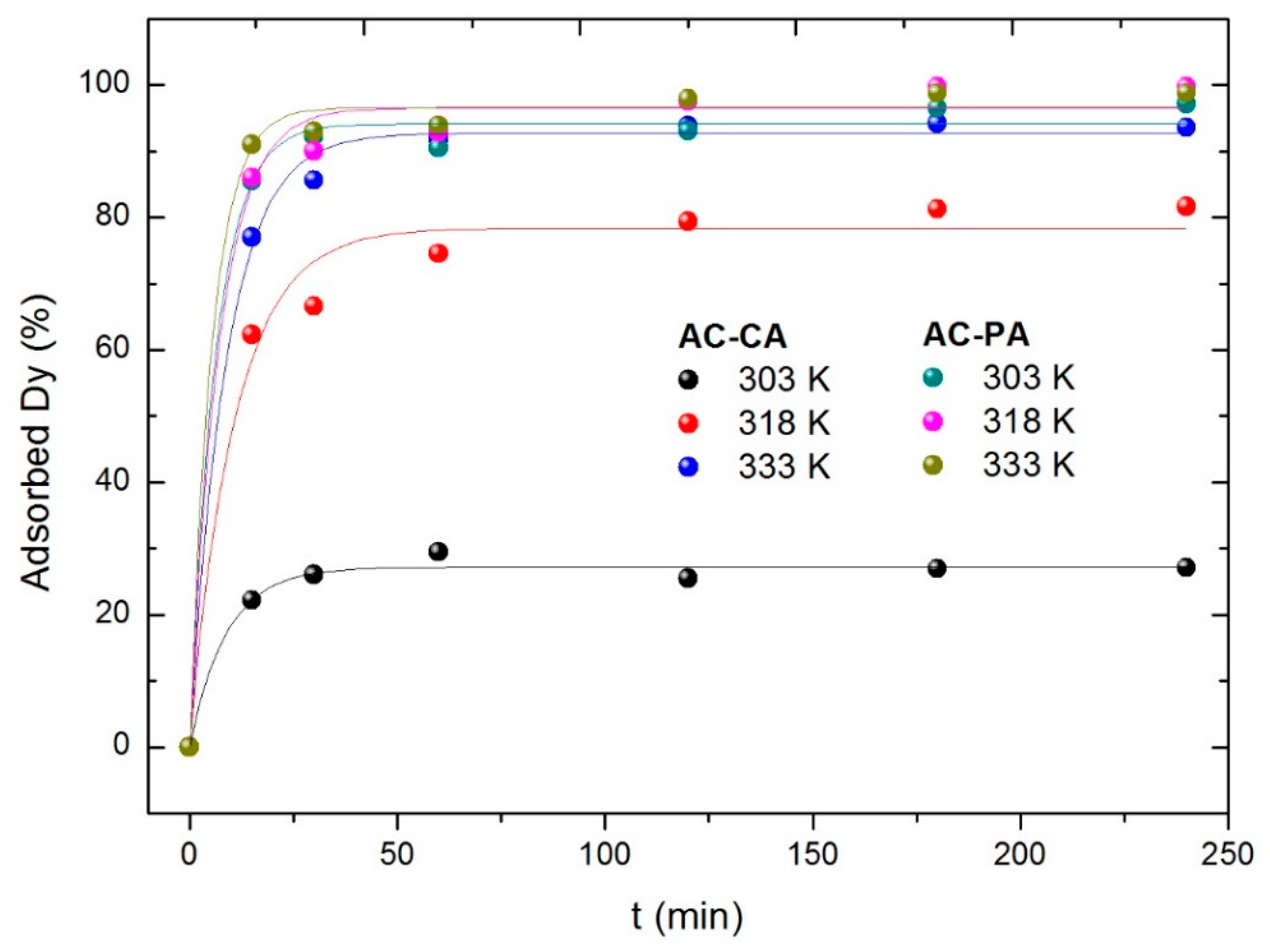


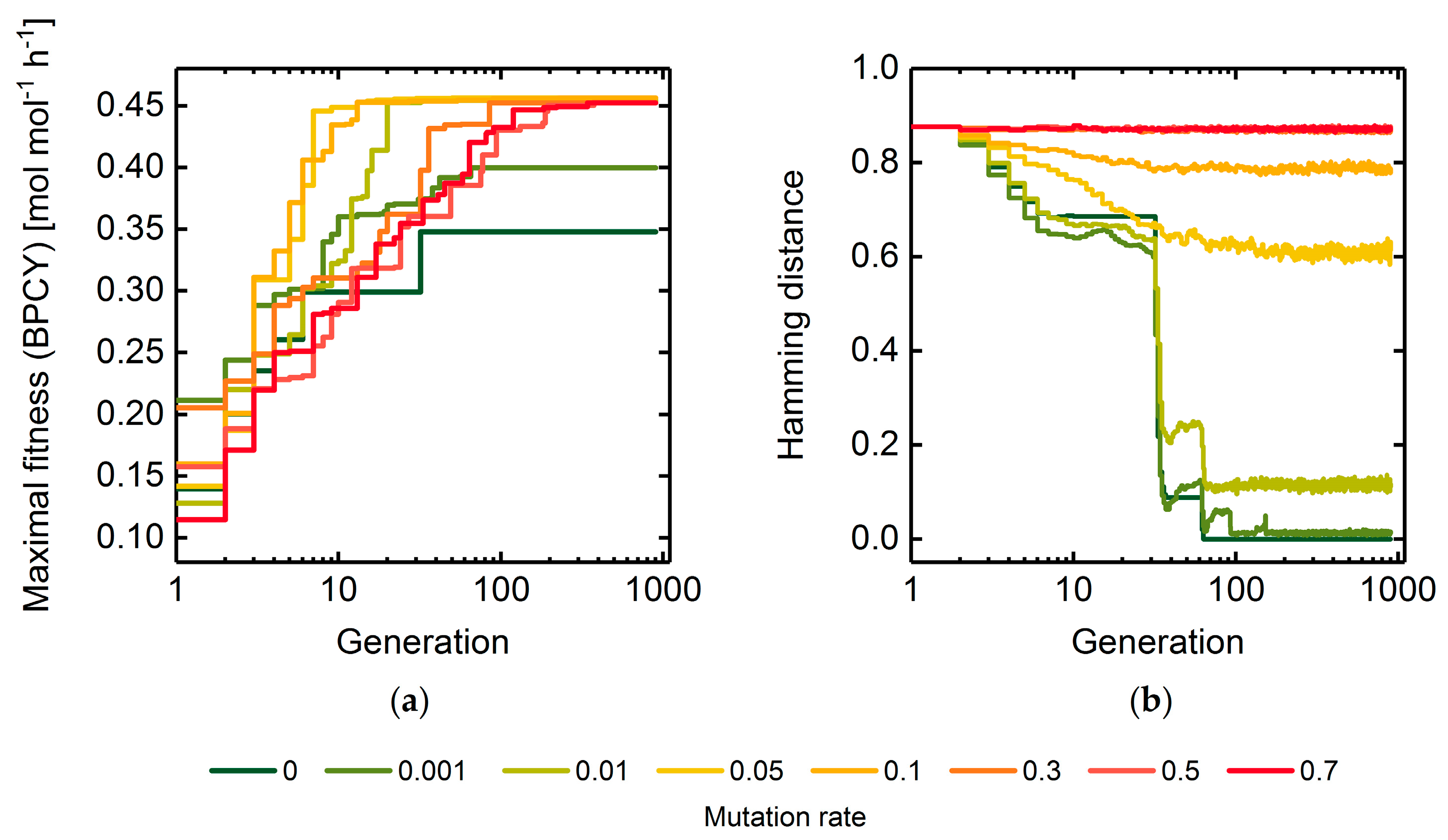



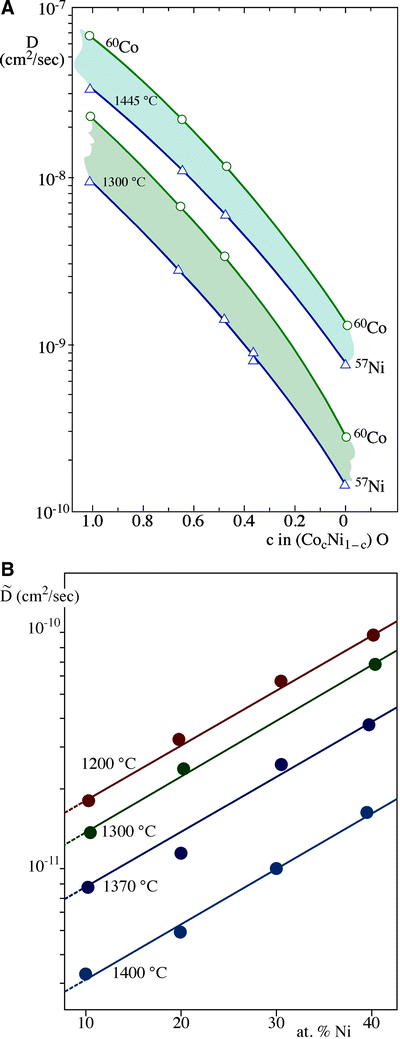

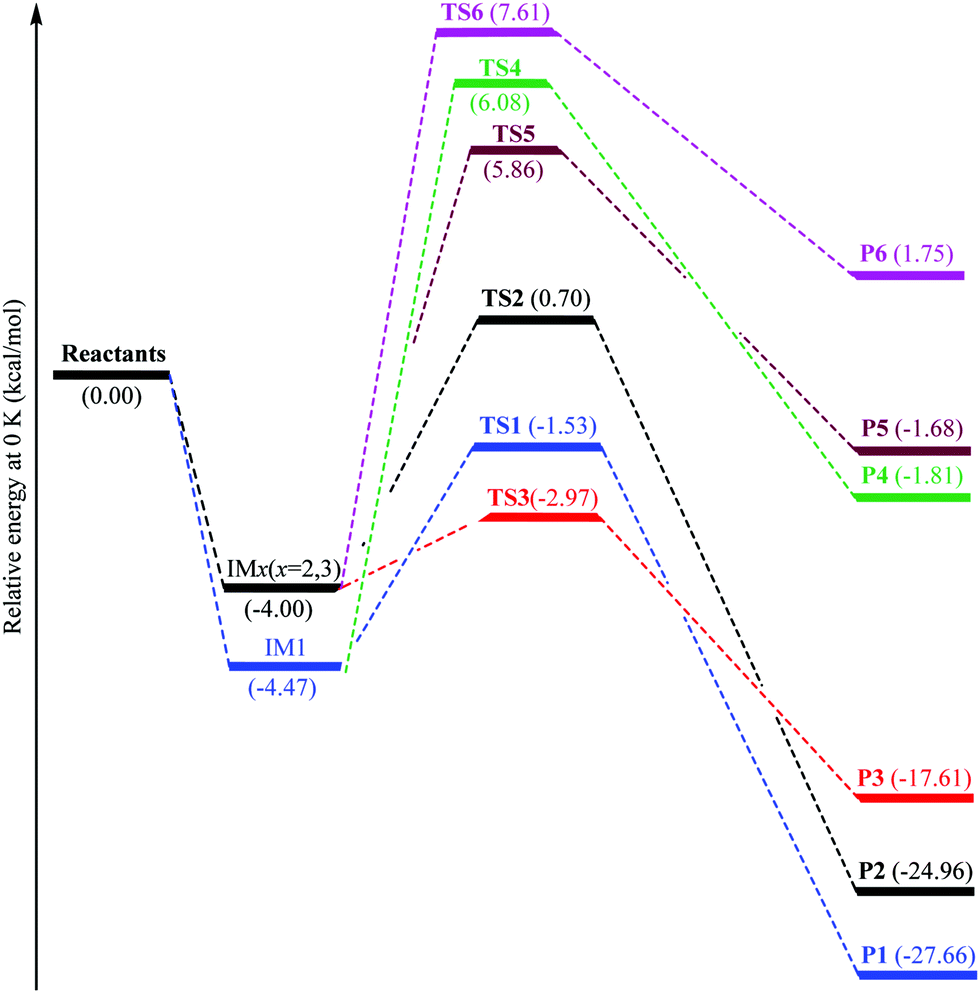
0 Response to "36 the diagram below represents a spontaneous reaction (δg"
Post a Comment