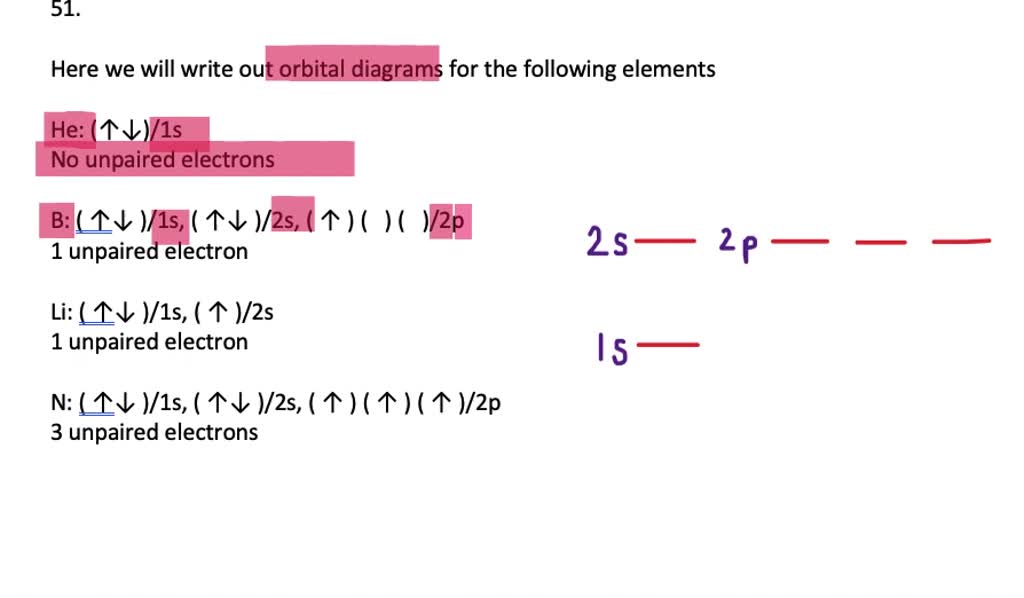
40 orbital diagram for mg
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
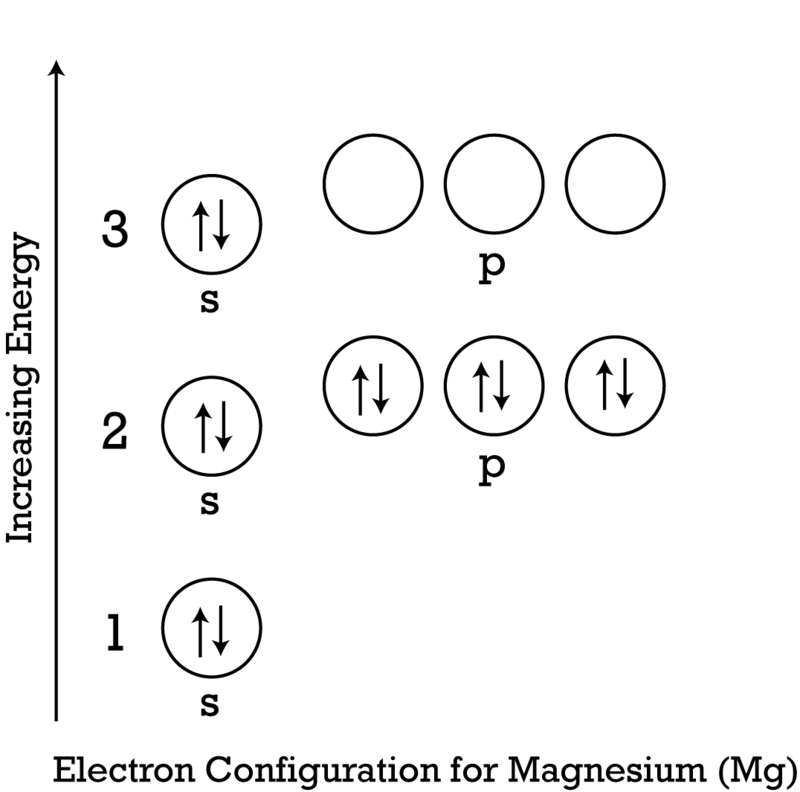
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.

Orbital diagram for mg
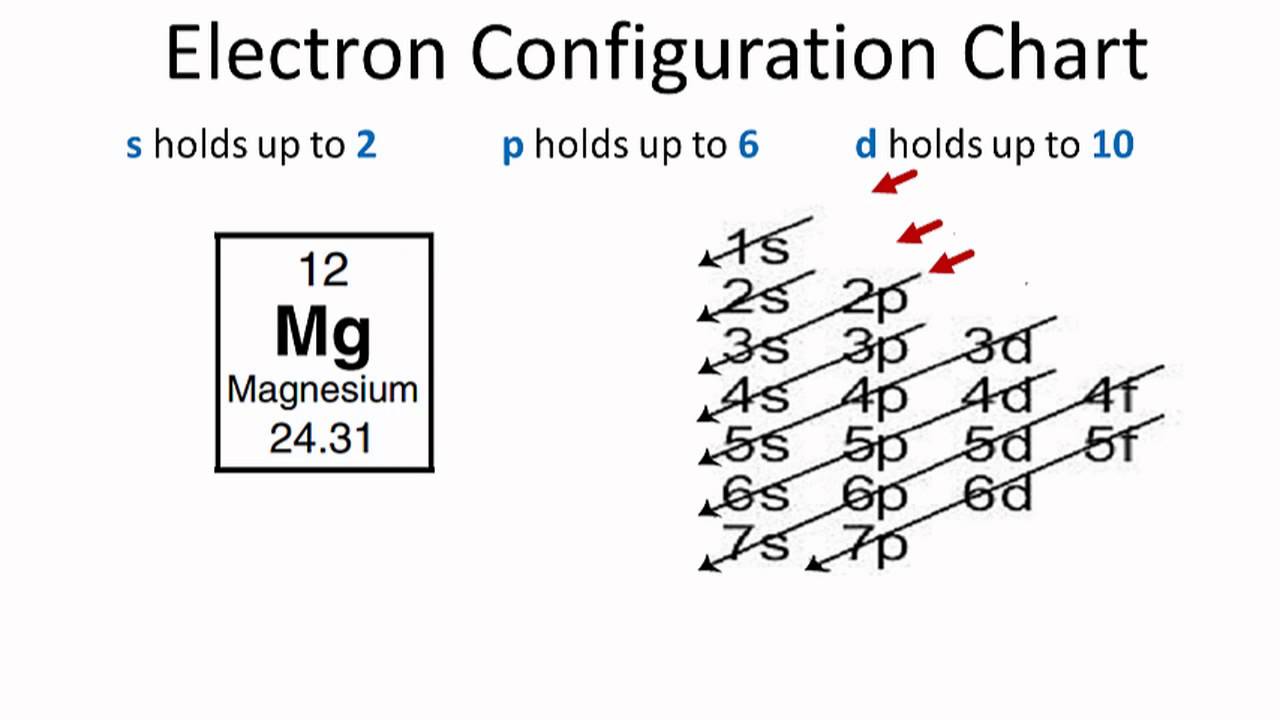
6 Aug 2021 — The number of electrons a shell or orbital can hold is based on 'The Pauli exclusion principle. To understand any element's electron ... The orbital diagrams for the eight elements are shown below. The electron configurations for the next eight elements are as follows: Na 1s 2 2s 2 2p 6 3s 1 Mg 1s 2 2s 2 2p 6 3s 2 Al 1s 2 2s 2 2p 6 3s 2 3p 1 Si 1s 2 2s 2 2p 6 3s 2 3p 2 P 1s 2 2s 2 2p 6 3s 2 3p 3 S 1s 2 2s 2 2p 6 3s 2 3p 4 Cl 1s 2 2s 2 2p 6 3s 2 3p 5 Ar 1s 2 2s 2 2p 6 3s 2 3p 6 Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
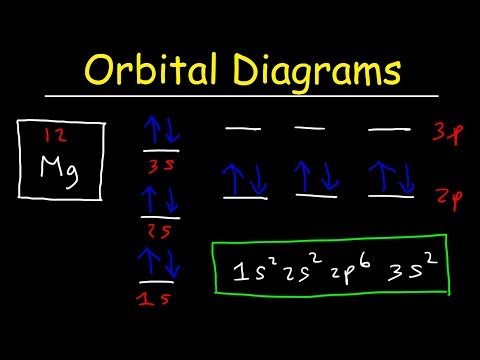
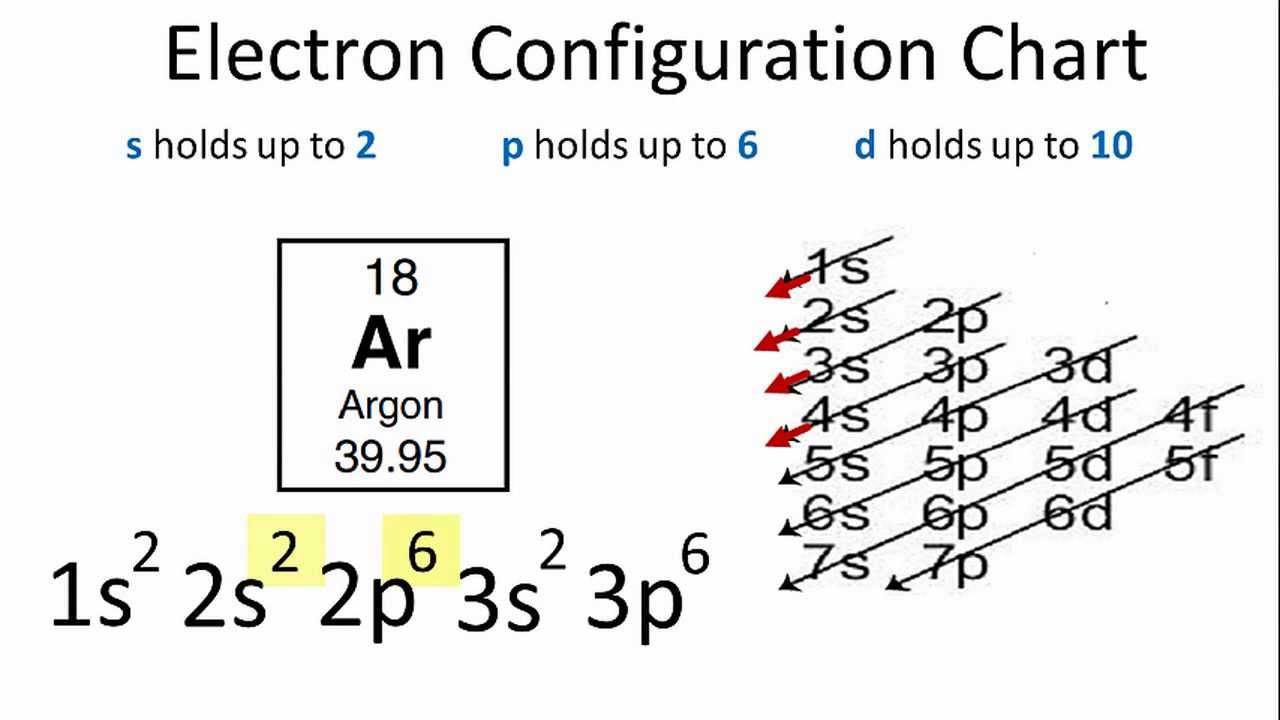
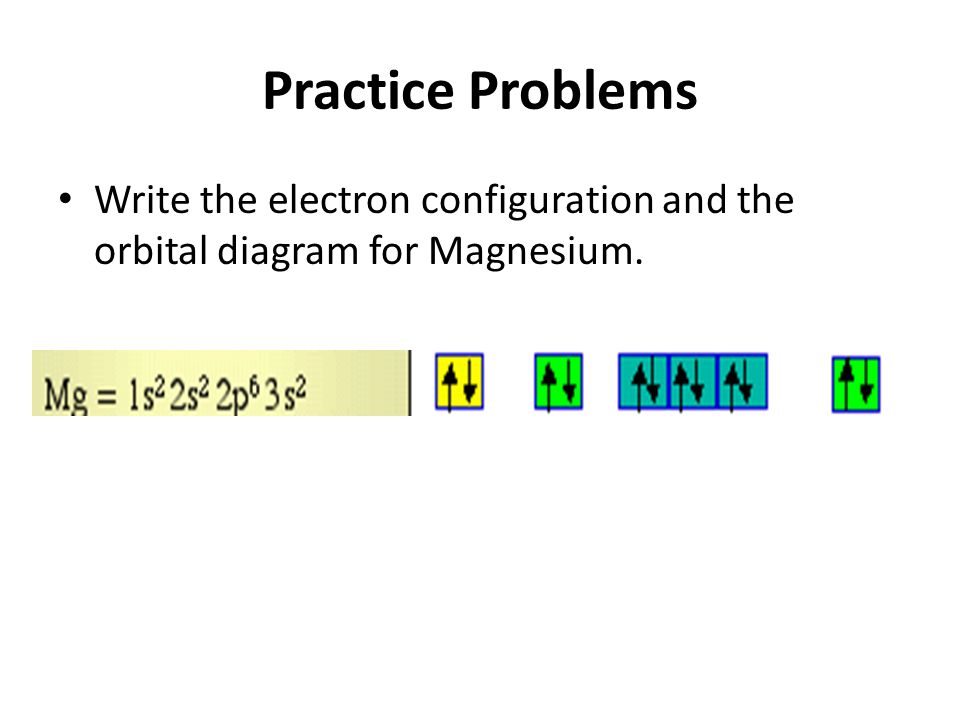
Orbital diagram for mg. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Similarly, what does mg2+ represent? Mg2+ is a Mg atom that now has the same number of electrons as a noble gas. It achiveved this by giving ... E. A 1s orbital can be represented as a two-dimensional circle centered around the nucleus of an atom. 3. When completing the orbital diagram for the element silicon, which of the following statements is correct? A. There are no electrons in the 3d sublevel. B. There are no electrons in the 3s sublevel. C. The 2p sublevel is not full. D. Show the orbital diagram for the element Mg. Orbital Diagram. The orbital Diagram is the most detailed form of the electron configuration for an element. The numbers relate to the energy level the ... Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
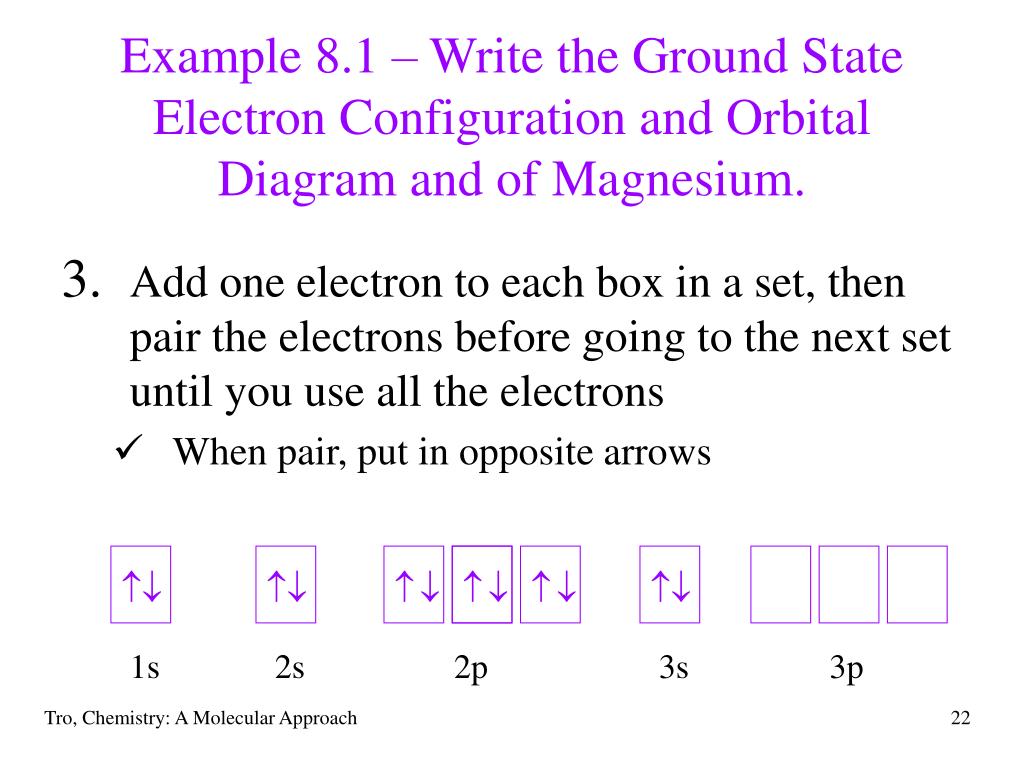
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The ...1 answer · Top answer: We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg.Recall that for a ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ... The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article. Also, period and group determination, valency and ... (Mg), and aluminum (Al) metals to form nitride compounds. 3Ca + N 2 (heat) → Ca 3 N 2 3Mg + N 2 (heat) → 2Mg 3 N 2 2Al + N 2 (heat) → 2AlN. But lithium(Li) metal reacts with N 2 at ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine 26 Jan 2021 — 1s2 2s2 2p6 3s2 is the Ground-state Electron Configuration of the Mg. ... Many other valence electrons of the element have been available here:. Transcribed image text: Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Orbital Diagram for Mg. quantum numbers and electron configurations there is only one orbital in the 2s subshell but there are three orbitals in the 2p subshell because there are three directions in which a p orbital can electron configurations how to write out the s p d f electron configurations for elements of atomic number z = 1 to 56 part 2 3 uses the rules on assigning electron ...
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s^2 2s^2 2p^1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As).
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
Step by Step: Electron Configurations and Electron Orbital Diagrams Electron Configurations Ex. 1) Mg: 1s 2 2s2 2p6 3s2 ↑↑↑ 1 = 1. st. layer (row #), s = orbital type , power of 2 = the 2 electrons in the 1s orbital **Move the Helium box next to Hydrogen (above Beryllium.) See the periodic table below.
What element does the following orbital diagram represent? Mg (magnesium). What element does the the following orbital diagram represent? V (vanadium). Solutions for Chapter 8 Problem 4SAQ. Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4.
Determine the electron geometry (eg) and molecular geometry (mg) of SiF4. A) eg=tetrahedral, mg=trigonal pyramidal B) eg=octahedral, mg=square planar C) eg=trigonal bipyramidal, mg=trigonal pyramidal ... Use the molecular orbital diagram shown to determine which of the following is most stable. A) C22⁺ B) N22⁺
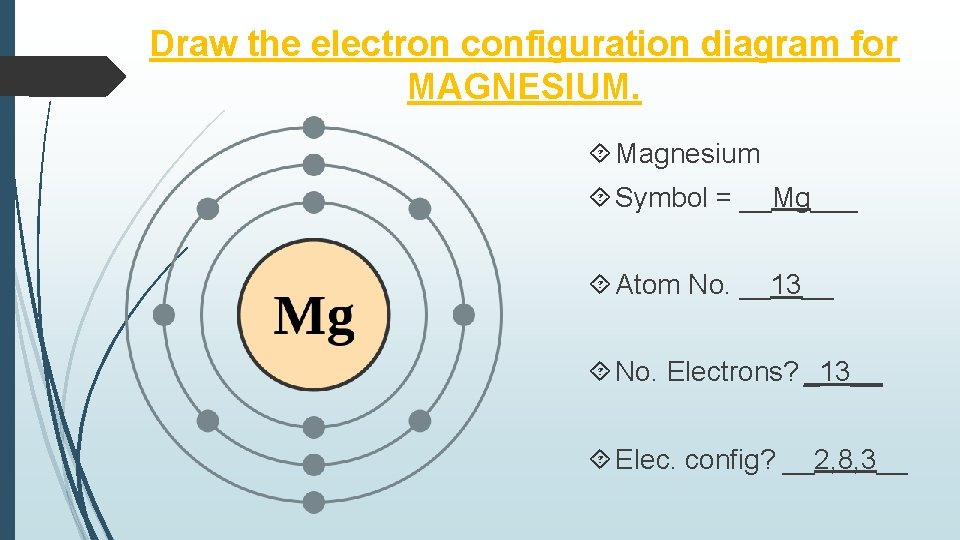
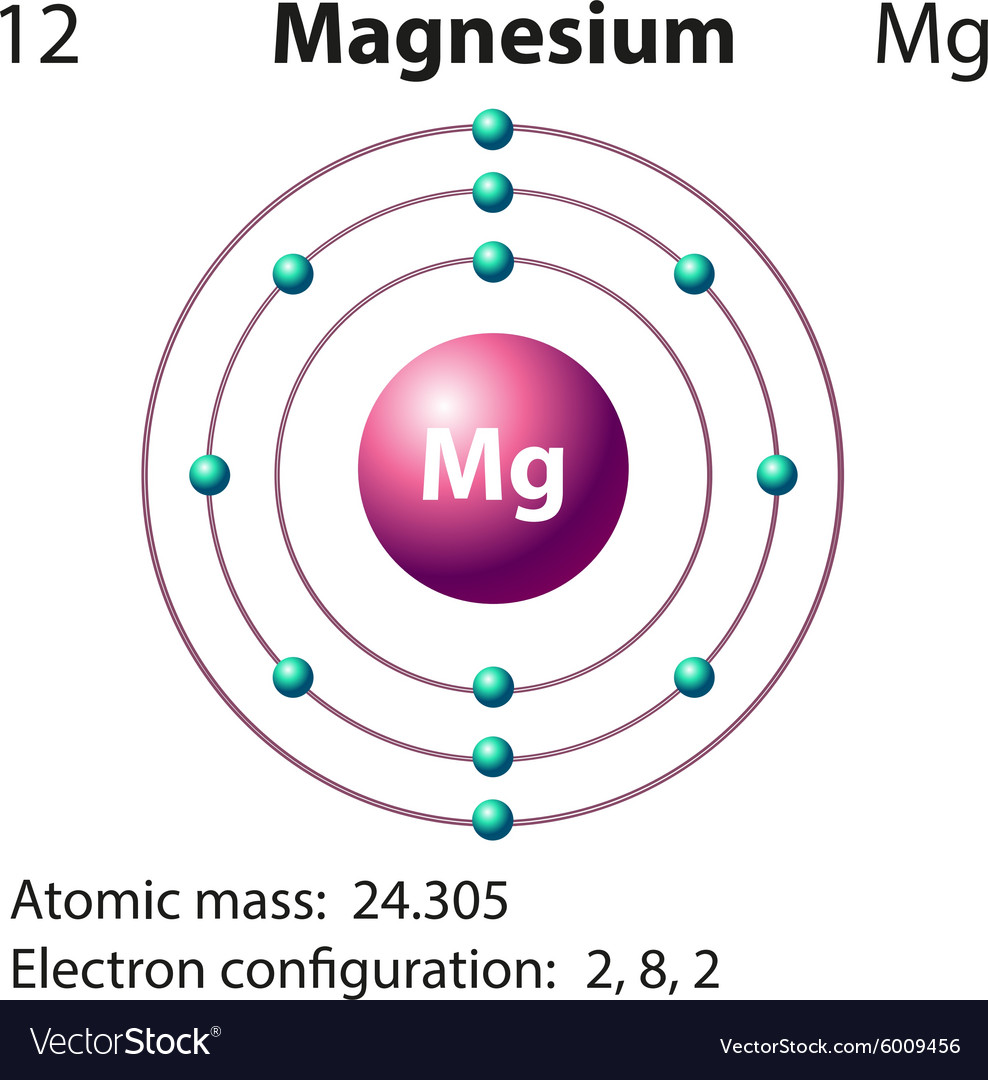
The atomic number of magnesium is 12. So, its electronic configuration is ( 2, 8 ). So, during chemical reaction, it gives up these 2 electrons in its outermost ...1 answer · 2 votes: This just shows energy levels so let's take this a step further. Atomic Electron Configurations ...
Magnesium (Mg) has an atomic mass of 12. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
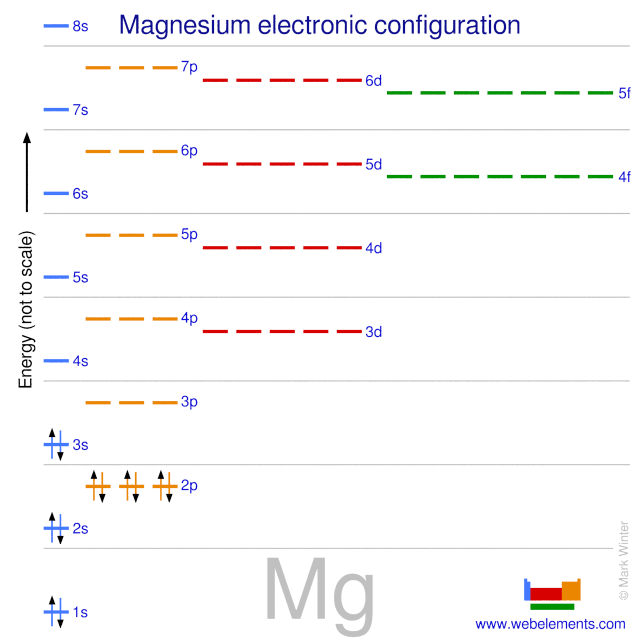
In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom (there are 12 electrons). The letter that designates the orbital type (the subshell, l), and; A superscript number that designates the number of electrons in that particular subshell. The notation describes the energy levels, orbitals and the number of electrons in each. the orbital ...
From the periodic table, we see that the atomic number of magnesium is 12. That is, a magnesium (Mg) atom has a total of twelve electrons. Step 2 - Step 2 is very important. In this step, the electrons of magnesium (Mg) have to be arranged. We know that magnesium atoms have a total of twelve electrons.
1 answerThe orbital notation for magnesium is: Mg_notation. Magnesium is in the third row and second column of the Periodic Table. This location on the Periodic...
Draw Orbit Structure And Electron Dot Diagram Of Naci Mgcl2 And Cao Sarthaks Econnect Largest Online Education Community
How does an orbital diagram to show the electron configuration for a neutral magnesium atom look. The p orbital can hold up to six electrons. In order to write the mg electron configuration we first need to know the number of electrons for the mg. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements ...
Then, since we are talking about "1 mol" of "Mg" atoms, multiply the number of MOs/atom by 6.0221413xx10^(23) to get: \mathbf("4 mol")s of "MOs/Mg atom" b) Okay, so some terminology from Band Theory. It's not too bad. THE HOMO-LUMO GAP The Fermi level is where the highest-occupied molecular orbital (HOMO) currently lies at "0 K".
What is the orbital diagram of MG? We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
HW #6 - Electron Configuration and Orbital Diagrams Directions: Please fill in the electron configuration for the following elements and then draw the orbital diagram configuration in the appropriate boxes. F Electron Configuration Mg Electron Configuration Orbital Diagram Configuration Orbital Diagram Configuration B Electron Configuration He
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
The orbital diagrams for the eight elements are shown below. The electron configurations for the next eight elements are as follows: Na 1s 2 2s 2 2p 6 3s 1 Mg 1s 2 2s 2 2p 6 3s 2 Al 1s 2 2s 2 2p 6 3s 2 3p 1 Si 1s 2 2s 2 2p 6 3s 2 3p 2 P 1s 2 2s 2 2p 6 3s 2 3p 3 S 1s 2 2s 2 2p 6 3s 2 3p 4 Cl 1s 2 2s 2 2p 6 3s 2 3p 5 Ar 1s 2 2s 2 2p 6 3s 2 3p 6
6 Aug 2021 — The number of electrons a shell or orbital can hold is based on 'The Pauli exclusion principle. To understand any element's electron ...

Electron Configurations Distributedexplains How Electrons Are Distributed Among An Atom S Orbitals Address Each Part Identifies Part Of An Electron S Address Ppt Download
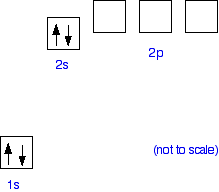
Solved A Write The Electron Configuration Draw The Orbital Diagram Determine The Distinguishing Electron And Determine The 4 Quantum Numbers For The Distinguishing Electron Of The Element Magnesium Mg Write Electron Configurations As
Solved Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg Mg Drag The Appropriate Labels To Their Res Course Hero

Write The Electron Configuration For Magnesium A 12 And Potassium A 19 Draw Their Orbital Brainly Ph
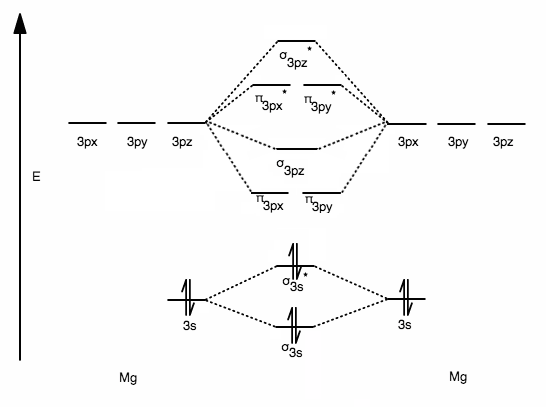
What Fraction Of The Orbitals In 1 Mol Of Mg Atoms In A Metallic Network Are Occupied At 0 K Socratic

















0 Response to "40 orbital diagram for mg"
Post a Comment