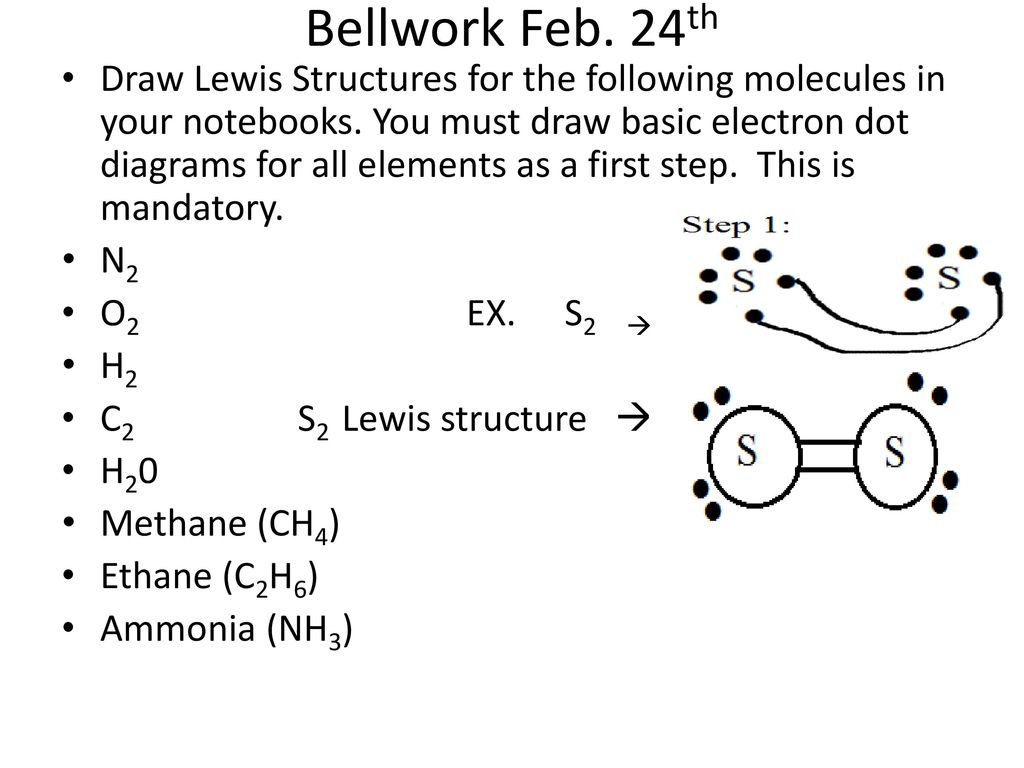
37 lewis dot diagram for c2h6
The C2H6 Lewis structure has a. H- O H • Electron Dot Diagrams show the electrons involved in the bonds of compounds.: 1 pair of C C. Electron Dot Diagrams C2H H H H C C.H All.The first step in drawing the Lewis dot structure for ethane (C_2H_6) is to determine how many valence electrons are available for the molecule. For ethane (C2H6), what is the correct framework of the Lewis structure? The central atoms consist of two carbons. How many valence electrons should you use to draw a Lewis structure of ammonia (NH3)?
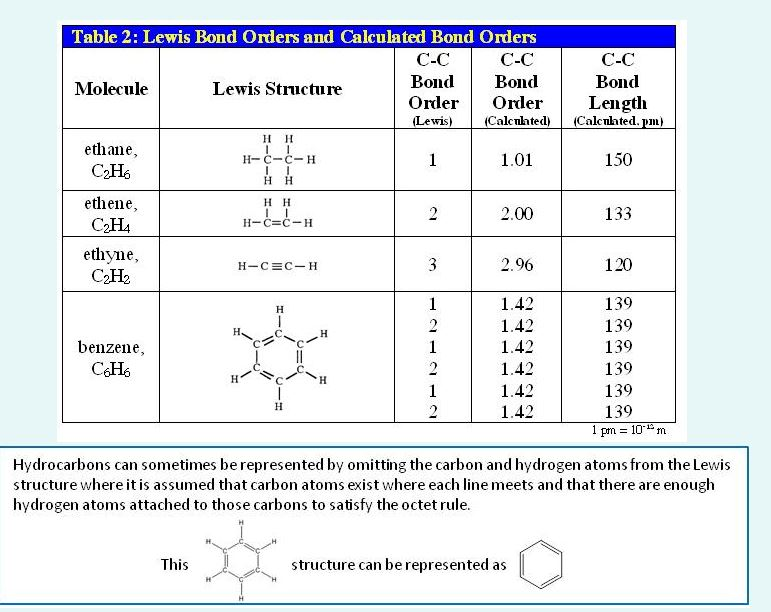
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Lewis dot diagram for c2h6
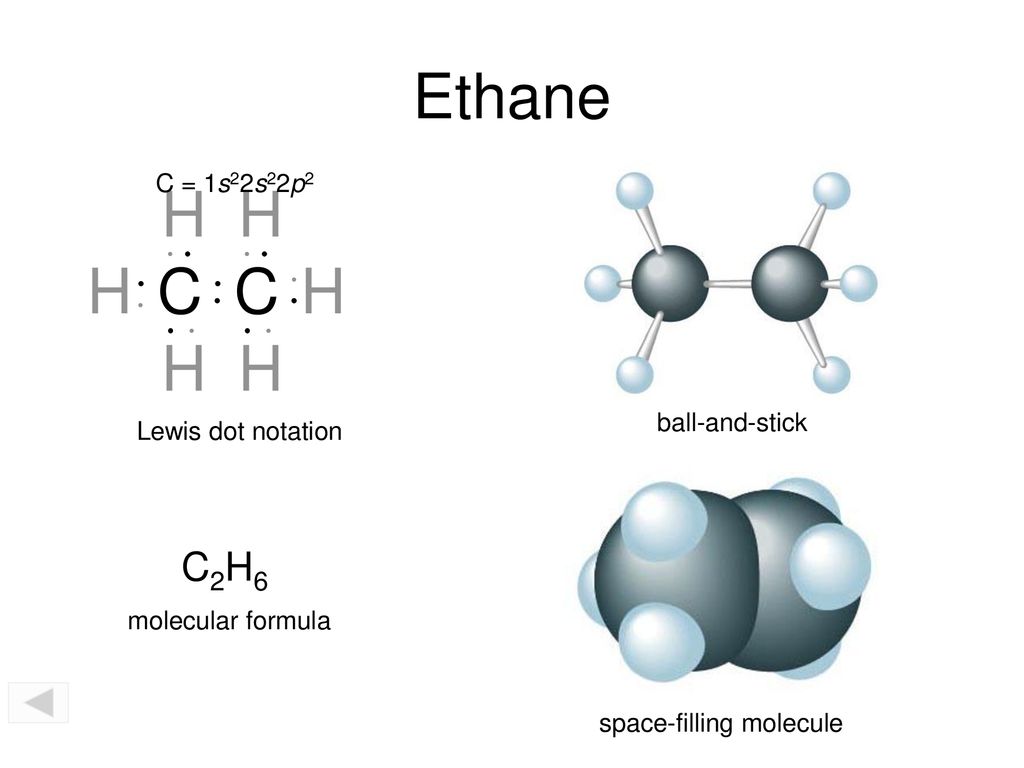
C2H6 lewis structure: Ethane Hybridization, Molecular Geometry and shape. Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. A step-by-step explanation of how to write the Lewis Dot Structure for C2H6 (Ethane)For the C2H6 Lewis structure, calculate the total number of valence elect... To determine the polarity of Ethane, we will first need to look at is Lewis Structure. We have also shared a detailed blog on the Lewis Dot Structure of Ethane previously which you can check out if you want to find out the number of valence electrons and the process of making C2H6 Lewis Structure step-by-step.. When we say it has the most simple structure, it is because only two types of atoms ...
Lewis dot diagram for c2h6. 100% (14 ratings) Transcribed image text: Draw the Lewis structure for ethane (C2H6). Be certain you include any lone pairs. Ć C C IT x s ?. Sometimes a protein is represented simply as a dot, as shown here for insulin. A simple shape is used here to represent a generic enzyme because the diagram focuses on enzyme action in general. 2. Draw a simple version of lysozyme that shows its overall shape, based on the molecular models in the top section of the figure. 3. Why is it unnecessary C2H6 Lewis Structure Lewis structure helps with understanding the placement of atoms in the structure along with its valence electrons. And then Hydrogen group 1 one valence electron. Drawing C is a Lewis electron dot structure for methane. C Group 4A 4 valence electrons likes 4 bonds H Group 1A 1 valence electron only forms 1 bond 81 333 ratings. According to the C2H4 lewis dot structure, carbon is the central atom and each carbon is attached to three atoms( 1 Carbon + 2 Hydrogen). Also lone pair present on the carbon is zero. So, H = 3 + 0 = 3 is the hybridization number for C2H4. Therefore hybridization of C2H4 is Sp². Note: Each carbon in the C2H4 Lewis structure contains Sp² ...
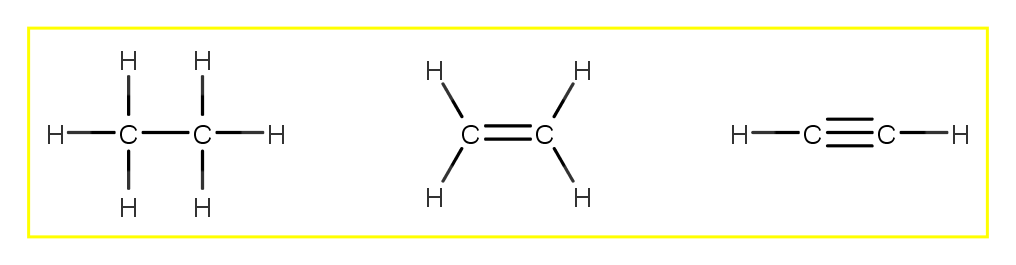
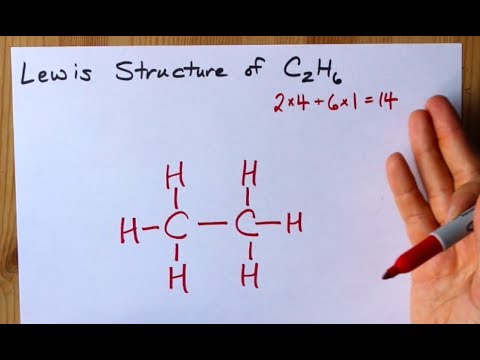
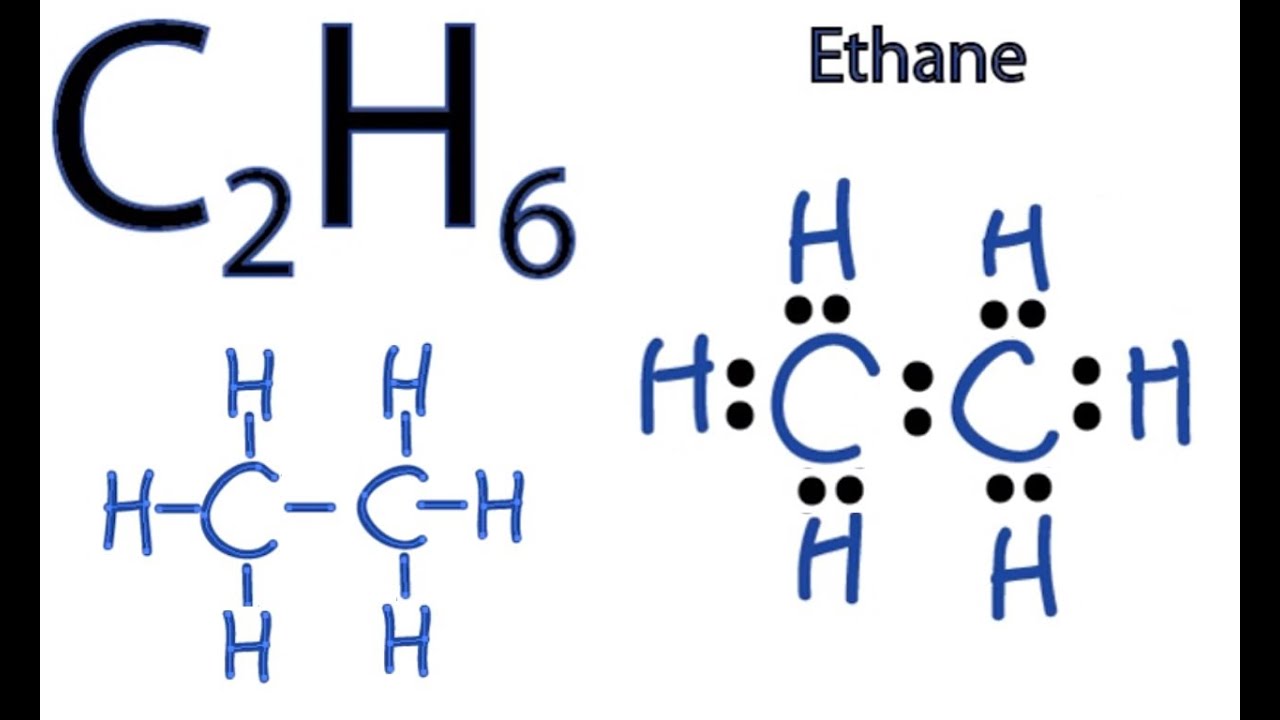
Lewis dot structure of C 2 H 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C:4x2=8 H:1x4=4. Total= Chemical Bonding: Lewis Dot Structure for C2H4 (6 of 6) Watch the video of Dr. B. drawing the Lewis dot structure for C 2 H 4 (ethene) and answer the questions below. Let's do the Lewis structure for C2H6, ethane. On the periodic table, Carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. So let's multiply that times 2. And then Hydrogen, group 1, one valence electron; we have 6, multiply that by 6, for a total of 14 valence electrons to work with. The first step in drawing the Lewis dot structure for ethane (C_2H_6) is to determine how many valence electrons are available for the molecule. Since C has 4 valence electrons, and each H atoms contributes 1 valence electron, the total number of electrons will be 2*4 + 6*1 = 14 "e"^(-) This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons, either through ... Use information from step 4 and 5 to draw the lewis structure. Lewis dot structure of C 2 H 6. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C:4x2=8 H=1x6=6. Total=14
The Lewis Dot Structure for C 2 H 6:. The Lewis dot structure for C 2 H 6 (ethane) shows the arrangement of valence electrons in the molecule, resulting from the formation of chemical bonds ... Nick Mirasol's Lewis Dot diagram video for C2H6 (Ethane) and IO3- (Iodate). C2h6 Lewis Dot Structure - Drawing Easy - https://draweasy9.blogspot.com/ 97PE. 98PE. 99PE. Draw the Lewis structure for C 2 H 6. Step-by-step solution. 100% (3 ratings) for this solution. Step 1 of 5. Lewis structure is the drawing that shows how electrons are shared in a molecule. This is represented by dots around the symbol of an atom of the element and a line between the atoms depicts the shared pair of electrons.
What is the Lewis structure for C2H6? This means that the Lewis dot structure for C2H6 must account for 14 valence electrons, either through bonding between atoms, or through lone pairs. So, the two C atoms are placed in the center of the molecule.
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.

Ethane Molecule C2h6 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes
12. Which Lewis elecfron-dot diagram represents chloroethene? C) H o c— H B) D) C C:CI: H D) :CI: 13. Given the formul o This compound is classified as H Given the sfructural formula: The compound represented by this formula can be classified A) an aldehyde C) an amine B) amide D) a ketone as an A) organic acid C) ester B ether D) aldehyde 14.
what does the Lewis Dot Structure of Sr(CN)2 look like? ap chem. consider the molecules PF3 and PF5. b]is the PF3 molecule polar or is it nonpolar. explain C] on the basis of bonding principles, predict whether each of the following compounds exists. In each case explain your prediction.
C2H6 Lewis Structure. Lewis structure is a 2D representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. It is based on the octet rule i.e. every atom tends to complete its octet ( 8 electrons) either by gaining or losing electrons except Hydrogen and Helium as they complete their duplet.
Lewis Dot of Ethane. C 2 H 6. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Ethane is a saturated hydrocarbon found in natural gas. At STP it is colorless, odorless gas.
Lewis structure helps with understanding the placement of atoms in. Lewis dot structure of C 2 H 6. Three are two ways to draw the lewis structure for c2h6o. What is the Lewis structure for C2H6. This means that the Lewis dot structure for C2H 6 must account for 14 valence electrons either through bonding between atoms or through lone pairs.
Answer (1 of 3): After determining how many valence electrons there are in C2H6, place them around the central atom to complete the octets. The C2H6 Lewis structure has a total of 14 valence electrons. Hydrogen (H) atoms always go on the outside of a Lewis structure.
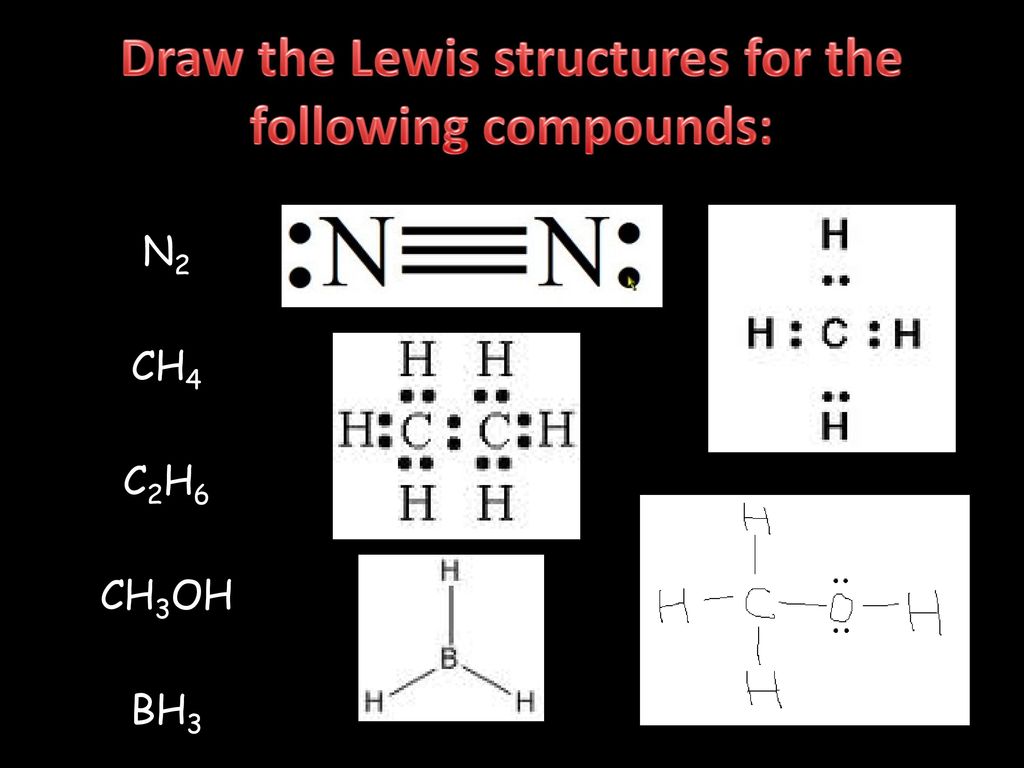
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
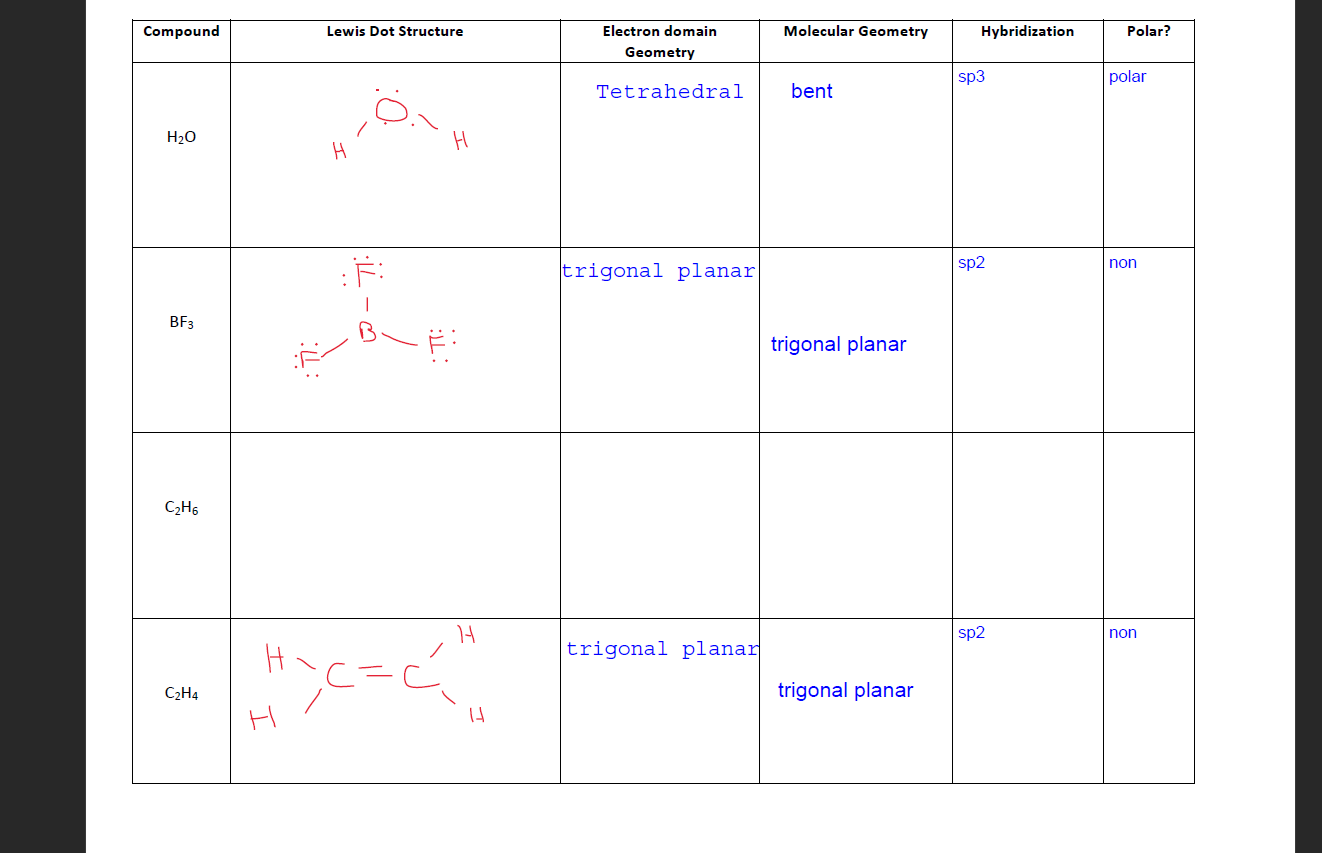
Lewis Dot Diagram For C2h6. Step method to draw lewis structure of ethane. Step 1: Find valence e- for all atoms. Add them together. C:4x2=8. H=1x6=6. Total= Step2: Find octet e- for. (a) Draw Lewis structures for ethane (C2H6), ethylene (C2H4), and acetylene (C 2H2). (b) What is the hybridization of the carbon atoms in each molecule?.
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
Nov 02, 2021 · In brief, the BeH2 molecule is a Lewis acid owing to the incomplete octet of the beryllium atom, which can be observed from its Lewis structure. The shape of BeH2 is linear with the sp hybridization of the beryllium atom. The Be-H bond is a polar covalent bond still the BeH2 molecule is nonpolar due to its linear shape and zero net dipole moment.
14+ C2H6 Lewis Structure. A lewis electron dot structure shows how the atoms of a molecule or an ion share their outermost electrons or valence electrons to form covalent bonds with each other. Both use all 20 valence. Double bond - Wikipedia from upload.wikimedia.org The exception, of course, being the…
To determine the polarity of Ethane, we will first need to look at is Lewis Structure. We have also shared a detailed blog on the Lewis Dot Structure of Ethane previously which you can check out if you want to find out the number of valence electrons and the process of making C2H6 Lewis Structure step-by-step.. When we say it has the most simple structure, it is because only two types of atoms ...
A step-by-step explanation of how to write the Lewis Dot Structure for C2H6 (Ethane)For the C2H6 Lewis structure, calculate the total number of valence elect...
C2H6 lewis structure: Ethane Hybridization, Molecular Geometry and shape. Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms.




















0 Response to "37 lewis dot diagram for c2h6"
Post a Comment