37 how is activation energy represented on an energy diagram
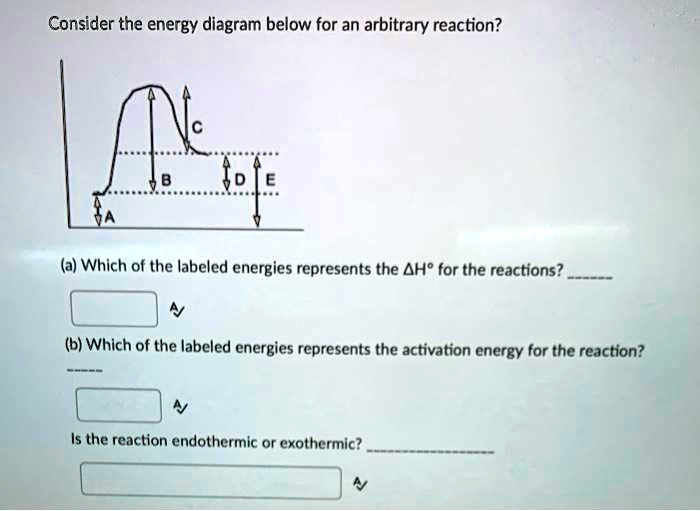
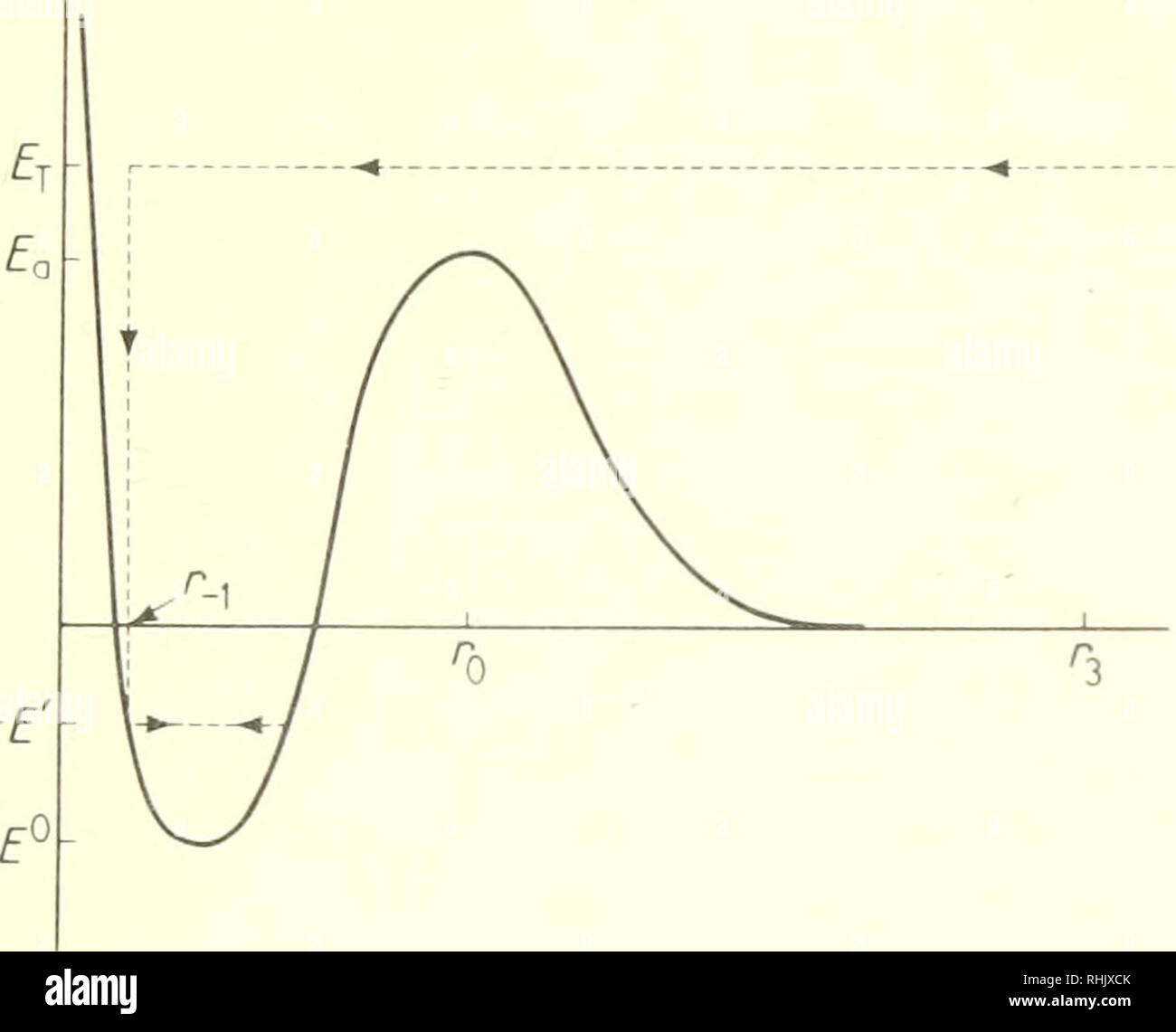
Solved Question 2 10 points On the accompanying energy ... The activation energy is represented by what on the diagram 3. Is this reaction shown on the diagram; Question: Question 2 10 points On the accompanying energy diagram shown below, answer the following questions reaction progress 1. Which represents the products and what is the energy of the products Give the answer to the nearest tens place. How is activation energy represented on an energy diagram ... Activation energy is the energy at the potential barrier between the two fields with low energy (at left and at right) representing the sum of energies of reactants and products; the reaction is...
PDF Potential Energy Diagram Notes - ms. adrangi's teaching site • Potential Energy diagrams represent changes in the potential energy of the reacting particles forming products when they are colliding. a) Activation Energy for the Forward Reaction o energy needed to form the activated complex. (or the energy needed to be overcome to form products).

How is activation energy represented on an energy diagram
Activation Energy - Definition, Formula, Diagram, Examples Activation energy is usually represented by E a and find from the Arrhenius mathematical formula, k = Ae -Ea/RT, where A = constant. It is obvious that the E a in the Arrhenius equation must have the units of energy. Generally, it is measured by the unit like joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). How can I represent the activation energy in a potential ... For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram. 28 Mar 2022 — How is activation energy represented on an energy diagram? Energy Reactants Products Reaction Progress o A. Activation energy is the final ...1 answer · 0 votes: d if not come back to meExplanation:
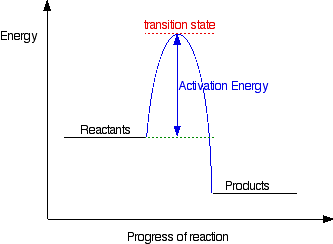
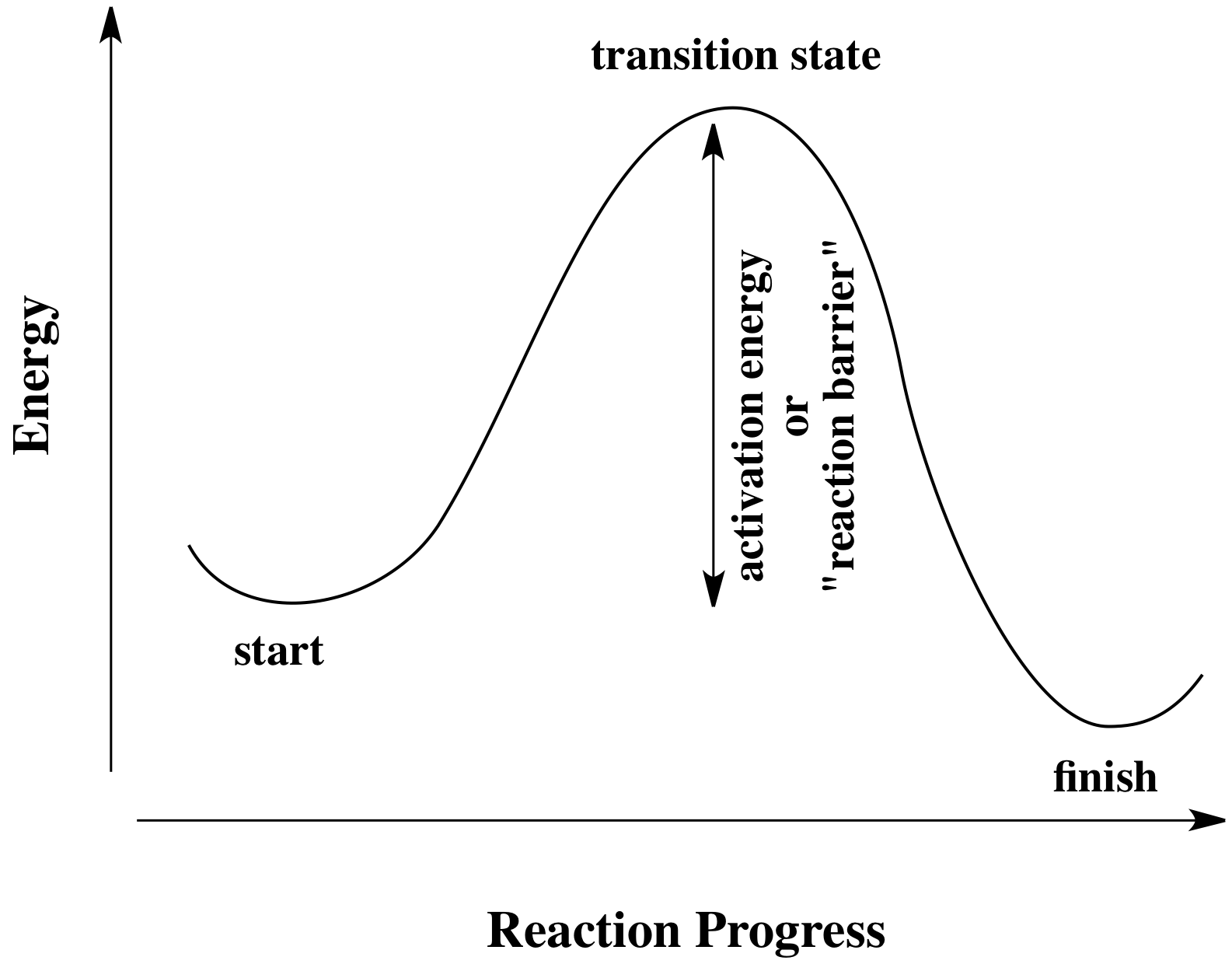
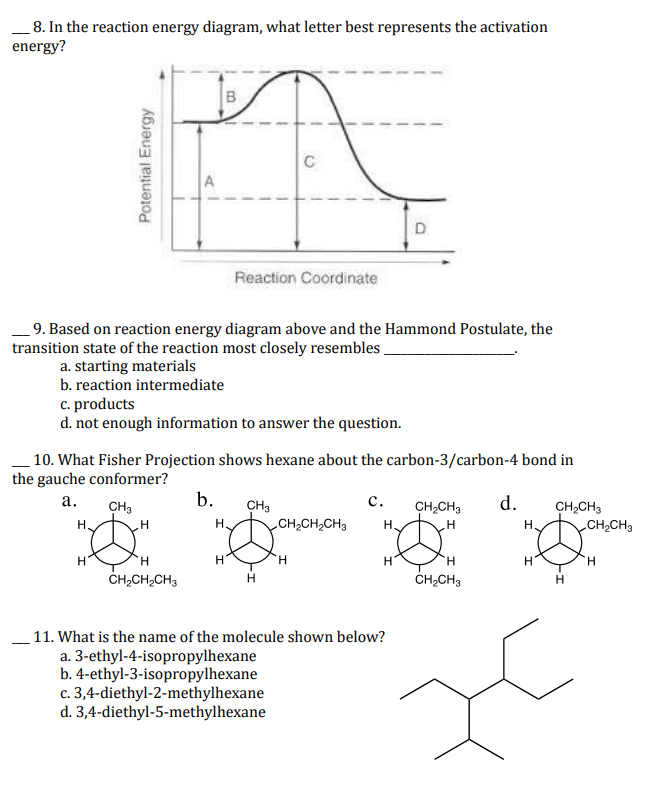
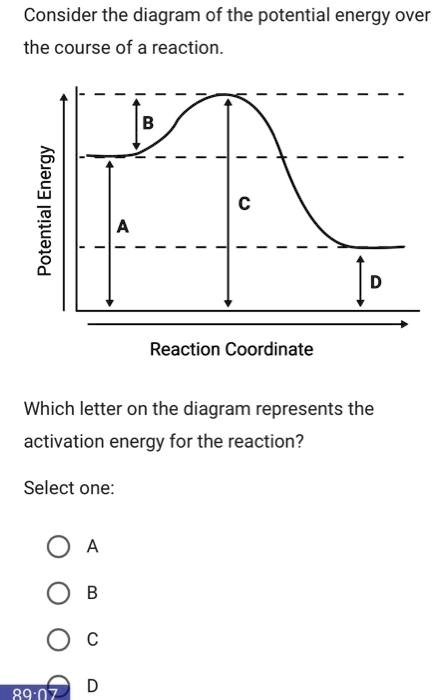
How is activation energy represented on an energy diagram. Potential Energy Diagrams - Kentchemistry.com In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. Effect of a Catalyst In the diagram, which letter represents the activation ... ajinems The letter that represents the activation energy from the diagram is letter A. A chemical reaction occurs only when there is collision between the particles of reactants. These colliding particles become activated with increased kinetic energy. Activation energy is the energy barrier that must be overcome before a reaction takes place. Answer Keus- POTENTIAL ENERGY DIAGRAM What letter represents the activation energy of the reverse reaction? –. 7. What letter represents the potential energy of the activated complex? 8. Is the ...8 pages PDF Chemical kinetics Name: Date - The Leon M. Goldstein High ... The accompanying diagram represents the energy changes that occur during the formation of a certain compound under standard conditions. According to Reference Table G, the compound could be A. C2H6(g) B. CO2(g) C. ICl(g) D. SO2(g) 10. A potential energy diagram is shown.
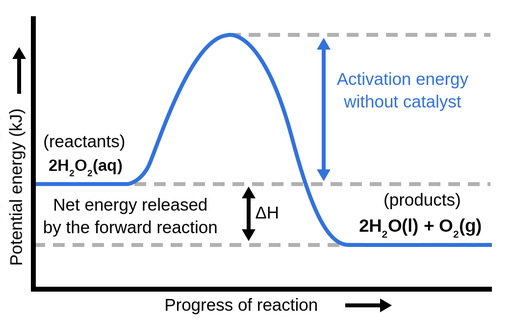
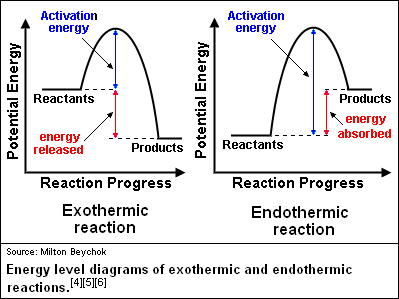
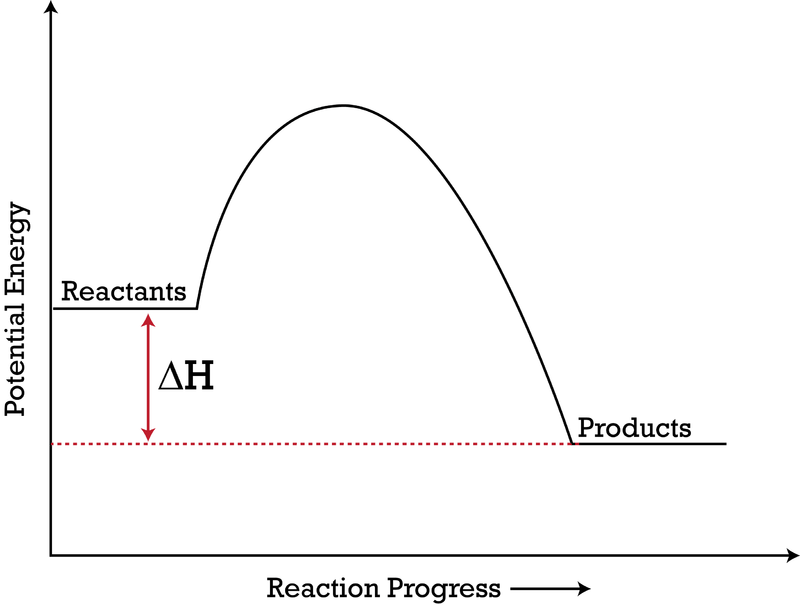
Solved Question 18 (1 point) Using the energy diagram ... The Activation Energy (EX) is represented by letter A Potential Energy D E Reaction Coordinate; Question: Question 18 (1 point) Using the energy diagram below, give the letter that corresponds to the description. Products are represented by letter A IN Question 19 (1 point) Using the energy diagram below, give the letter that corresponds to the ... 2 HI(g)→H 2(g)+ I 2(g) - Brainly.com The activation energy is the minimum energy that the reactants must possess to the able to form products. On the graph, it is denoted as Ea, the difference in energy between the peak of the curve and the emergy level of the reactants. So, a catalyst will cause this activation energy to be lowered to a new level of Ea₁. Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction. energy profiles - chemguide Diagrams like this are described as energy profiles.In the diagram above, you can clearly see that you need an input of energy to get the reaction going. Once the activation energy barrier has been passed, you can also see that you get even more energy released, and so the reaction is overall exothermic.
18.4: Potential Energy Diagrams - Chemistry LibreTexts Reaction profiles - Exothermic and ... - BBC Bitesize The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. Potential_Energy_Diagram_Extra_Practice (1).pdf - 1. Which ... Given the potential energy diagram representing a reversible reaction: The activation energy for the reverse reaction is represented by A) heat of fusion B) entropy of the system C) heat of reaction ionization energy diagram D) activation energy 4. How does activation energy play a role in reaction rates ... What does the activation energy for a chemical reaction represent quizlet? Activation energy is the energy absorbed before it can start a chemical reaction. the diagram in figure 13 shows the amount of energy the reactions starts and how much energy is being released or absorbed depending on the reaction that is occurring.
What is the activation energy on an energy diagram? For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram.
PDF Representing a Reaction with a Potential Energy Diagram diagram. The activation energy for the reverse reaction, E a(rev), is the difference between the product energy and transition state at the peak of the diagram. ... Draw a potential energy diagram that would reasonably represent this combustion reaction.
Which arrow represents the activation energy of the ... Given the potential energy diagram representing a reversible reaction: The activation energy for the reverse reaction is represented by A) heat of fusion B) entropy of the system C) heat of reaction ionization energy diagram D) activation energy 4.
What is an activation energy diagram in chemistry? - Answers Activation energy is the energy at the potential barrier between the two fields with low energy (at left and at right) representing the sum of energies of reactants and products; the reaction is...
Solved On the accompanying energy diagram shown below ... The activation energy is represented by what on the diagram catalyzed reaction 3. Is this reaction shown on the diagram exothermic or; Question: On the accompanying energy diagram shown below, answer the following questions 1. Which represents the products and what is the energy of the products Give the answer to the nearest tens place.
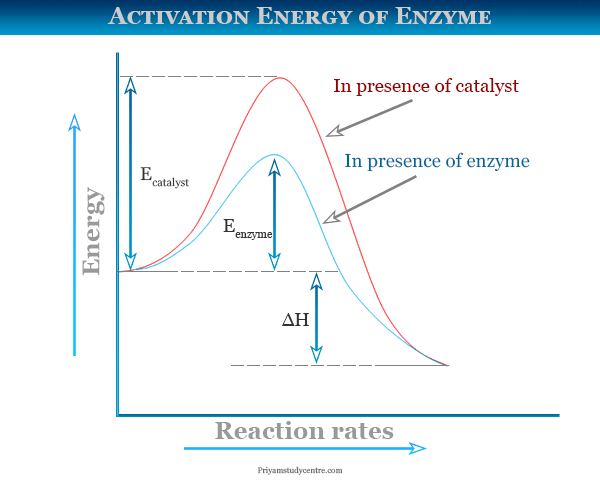
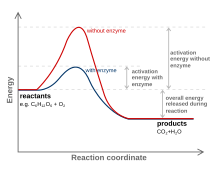
Activation Energy of Enzymes: Definition, Calculation ... The activation energy is the energy required to start a reaction. Enzymes are proteins that bind to a molecule, or substrate , to modify it and lower the energy required to make it react.
Learn About Activation Energy In Energy Diagram | Chegg.com The activation energy is represented by an energy diagram. In an energy diagram, the reaction progress is plotted on the x-axis and the potential energy is plotted on the y-axis. When the reaction begins the initial energy of the reactants is constant and then the energy starts increasing until maximum energy requirement is fulfilled.
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
Solved In the energy energy diagram of the following ... In the energy energy diagram of the following reaction. The activation energy of the determining step of the reaction is represented by the letter: Captionless Image a (red) c (brown) d (green) b (blue) D A b a с C ENERGIA d E e Transcurso de la reacción ; Question: In the energy energy diagram of the following reaction. The activation energy ...
28 Mar 2022 — How is activation energy represented on an energy diagram? Energy Reactants Products Reaction Progress o A. Activation energy is the final ...1 answer · 0 votes: d if not come back to meExplanation:
How can I represent the activation energy in a potential ... For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram.
Activation Energy - Definition, Formula, Diagram, Examples Activation energy is usually represented by E a and find from the Arrhenius mathematical formula, k = Ae -Ea/RT, where A = constant. It is obvious that the E a in the Arrhenius equation must have the units of energy. Generally, it is measured by the unit like joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).














0 Response to "37 how is activation energy represented on an energy diagram"
Post a Comment