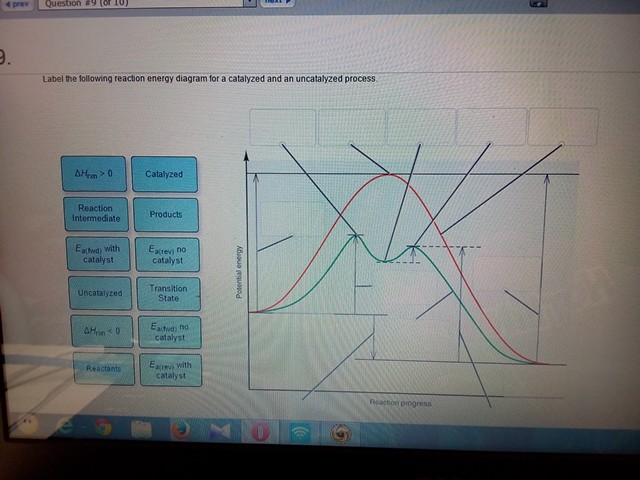
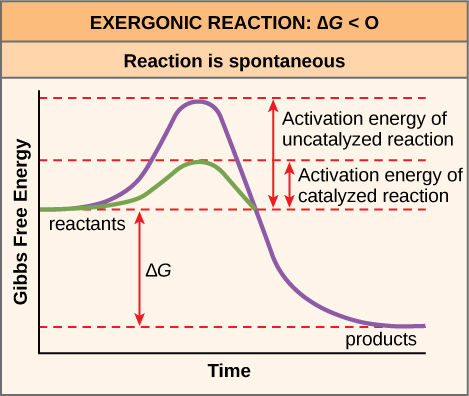
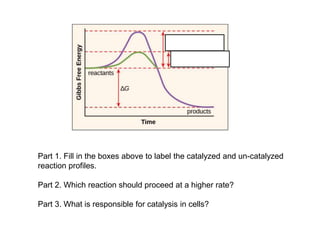
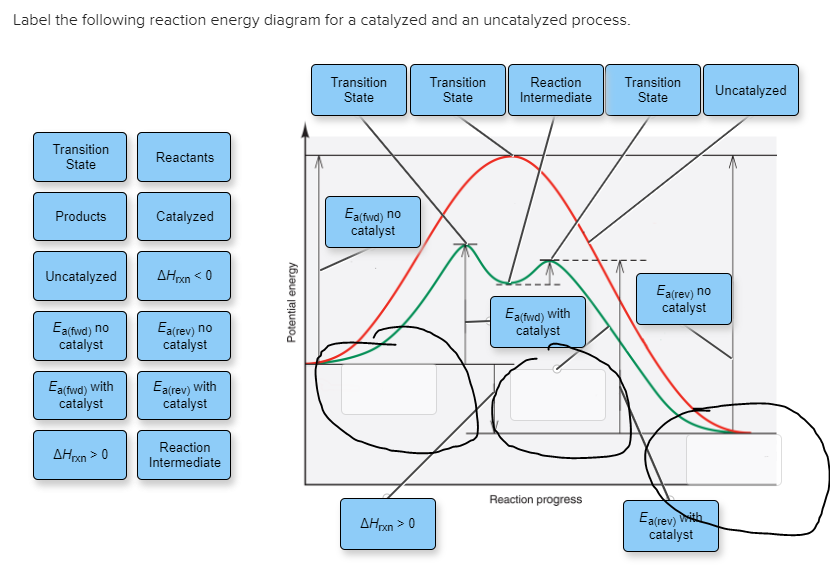
39 label the following reaction energy diagram for a catalyzed and an uncatalyzed process
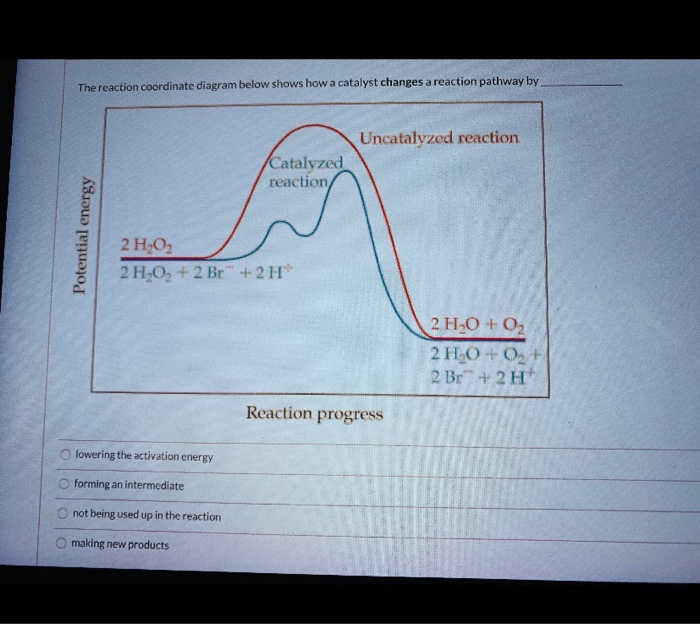
› 34302852 › essential_biochemistry(PDF) essential-biochemistry-third-edition-charlotte-w-pratt ... Academia.edu is a platform for academics to share research papers. Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows%(1). The decomposition of hydrogen peroxide is exothermic.
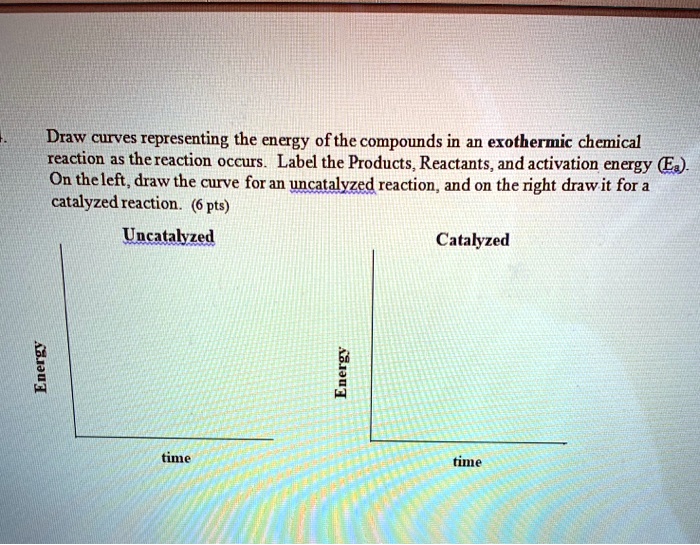
PDF ap07 chemistry q6 - College Board (e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction. One point is earned for an uncatalyzed reaction curve that must show that E a > 0 and ΔH < 0. One point is earned for a catalyzed reaction curve that must show E
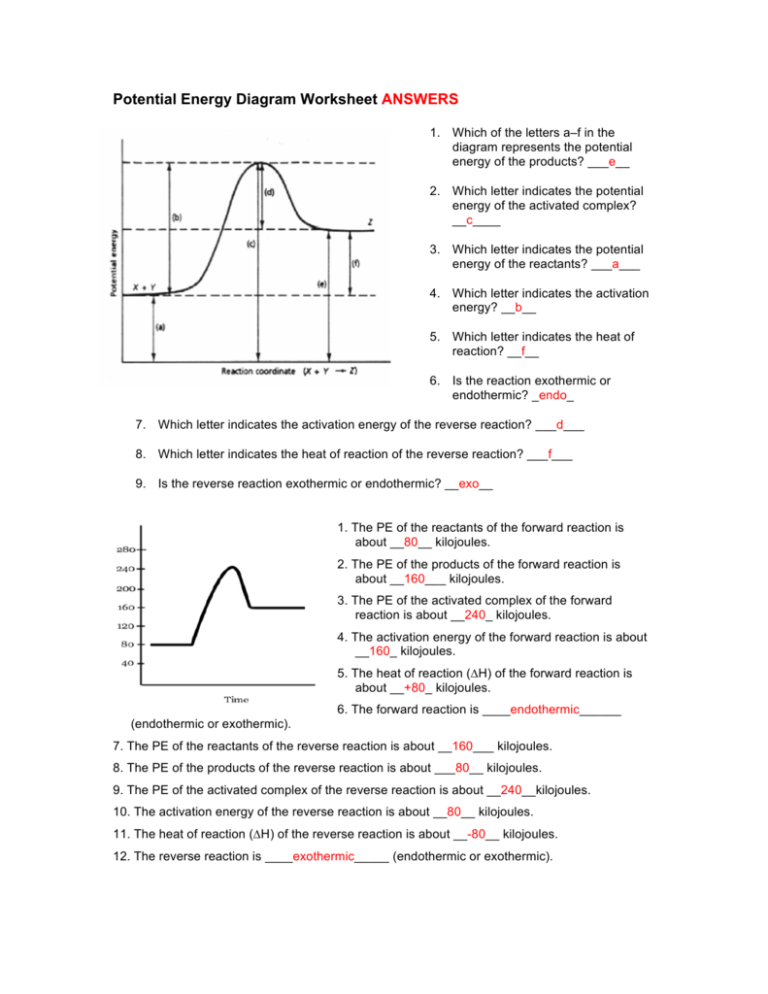
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process
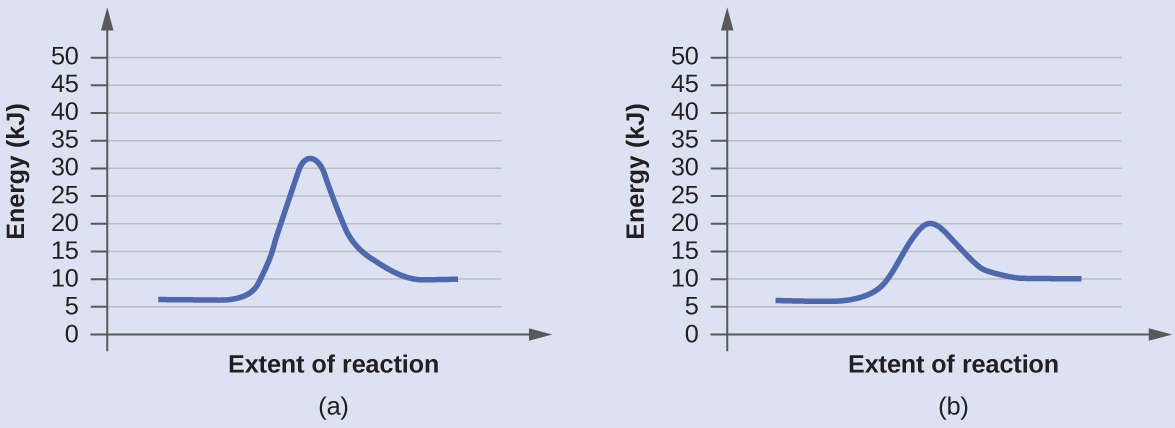
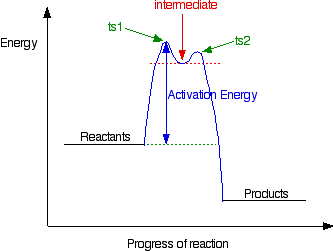
QUIZ 2 mega quizlet Flashcards | Quizlet - The type of reaction catalyzed by proteases. - An enzyme that is part of a pigment formation pathway and has a low-temperature optimum. - A protease enzyme with a low pH optimum. - The inhibitor that binds only to the ES complex and lowers the Vmax and KM. - The enzyme inhibition that can be overcome by increasing concentration of substrate. 40 label the energy diagram for a two-step reaction ... Label the energy diagram for a two-step reaction. Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Label ΔH as positive or negative. How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
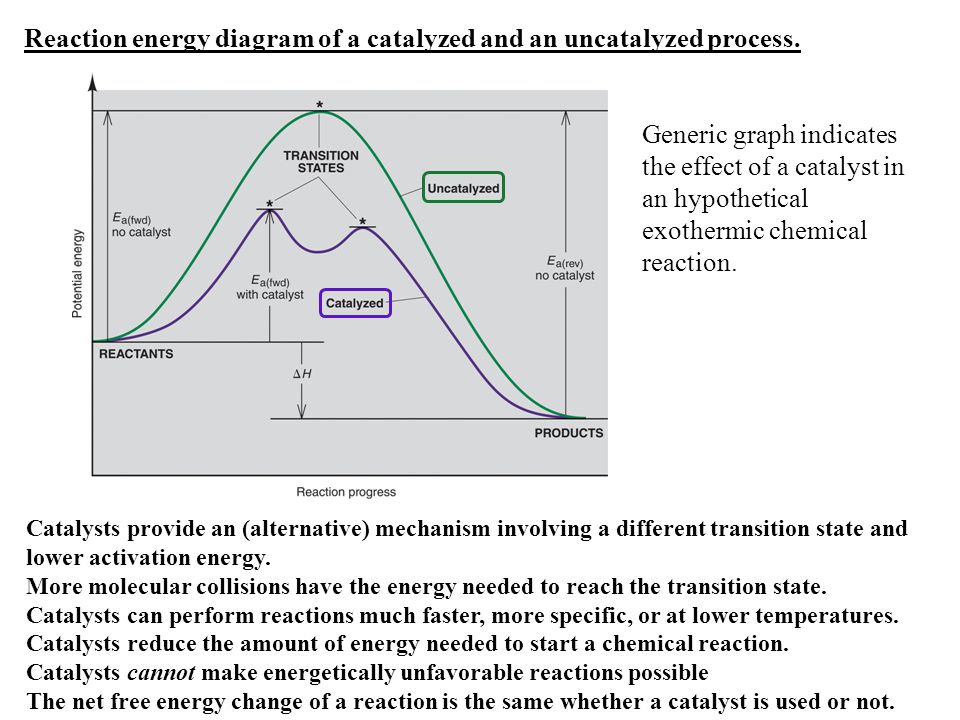
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. › document › 325957447Solution Manual Brady Chemistry 6TH Edition PDF | PDF | Ion ... This is an example of the Maillard reaction describing the chemical reaction of sugar molecules and amino acids. (d) Chemical change. Wine contains ethanol which can be converted to acetic acid. (e) Chemical change. Concrete is composed of many different substances that undergo a chemical process called hydration when water is added to it. 7 ... 12.7 Catalysis - Chemistry 2e | OpenStax The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction mechanism with a lower activation energy. 33 Label The Following Reaction Energy Diagram For A ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Label the energy diagram and answer the question that follows. The solid line in the energy diagram represents changes in energy as the reactant is converted to product under standard conditions. Which equation below indicates how the presence of an enzyme affects the activation energy of the reaction catalyzed versus uncatalyzed. The mechanism may be different for a catalyzed reaction reaction vs the ... Solved Label the following reaction energy diagram for a ... Best Answer. This is the best answer based on feedback and ratings. 90% (40 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Previous question Next question.
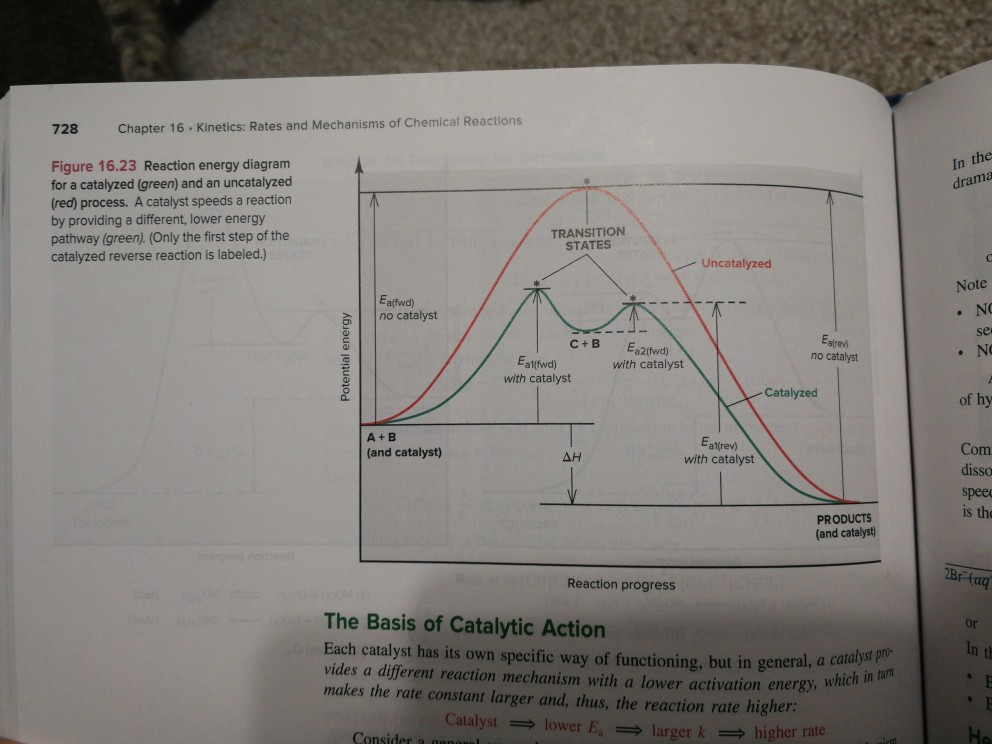
Label the following reaction energy diagra... | Clutch Prep We're asked to label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Recall that a catalyst speeds up a reaction by lowering the activation energy needed. This means that: The red line corresponds to the uncatalyzed reaction (steeper hill) The green line corresponds to the catalyzed reaction (lower hill) 12.7 Catalysis - Chemistry A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. achieverstudent.comAchiever Student: We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations. en.wikipedia.org › wiki › Cold_fusionCold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion.
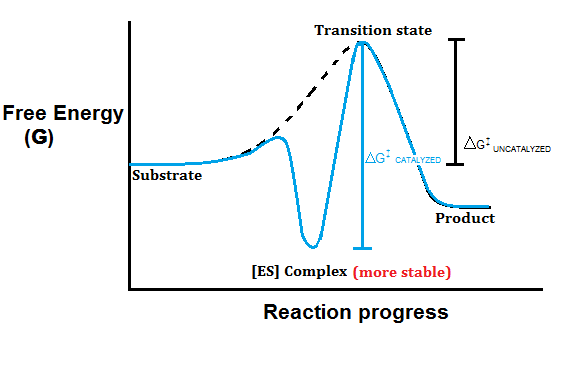
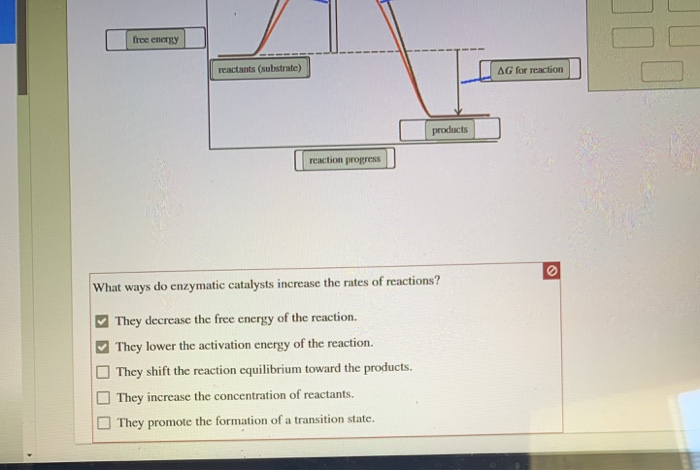
Catalysis | Chemistry for Majors - Lumen Learning The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction mechanism with a lower activation energy. bio.libretexts.org › Courses › University_ofW_2022_Bis2a_Igo_Reading_07 - Biology LibreTexts Interpret a biochemical transformation and predict whether or not the reaction is spontaneous by using a Gibbs enthalpy (energy) reaction coordinate diagram. Describe the concept of equilibrium in the context of reaction coordinate diagrams. Describe mechanisms used by enzymes to lower the activation energy and increase rates of reaction. Energy Changes in Catalytic Reactions - QS Study A catalyst provides a different route with lower activation energy. Fig: Energy diagram for unanalyzed and catalyzed reactions. In Figure, the unanalyzed reaction is shown as a single step with high energy of activation. The catalyzed reaction is shown as a two-stage process with lower activation energy and is, therefore, faster. Energy diagrams for enzyme‐catalyzed reactions: Concepts ... The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the same process.
Solved Label the following reaction energy diagram for a ... Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea(fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea(rev) no catalyst Ea(fwd) no catalyst Ea(rev)
› 34415930 › Lippincotts(PDF) Lippincott's Illustrated Reviews ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Chapter 8 BIOCHEM Flashcards - Quizlet Enzymes also increase the rate of a biological reaction compared to the rate of the uncatalyzed reaction. Label the energy reaction graph for the following reaction showing the energy profile for a catalyzed and an uncatalyzed reaction. Ea, rxn not cat by enzyme, rxn cat by enzyme, energy released by rxn.
Use the following potential energy diagram to answer ... (1 mark) d) Label the graph with "RI" to indicate the location of the reaction intermediate(s). (1 mark) e) If the above graph represents a catalyzed proposed reaction mechanism, use a dashed line or another colour, to sketch a possible curve that would represent the route for the uncatalyzed overall reaction.
Label The Following Reaction Energy Diagram For A ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.Catalysts lower the activation energy for reactions. Figure 1 shows how an enzyme (or any other catalyst) we can express this reaction by the following chemical equation the line labeled uncatalyzed reaction in figure 1 represents changes in energy that take place in the reaction without a catalyst.
Label the following reaction energy diagram for a ... Q&A Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Answer All the labeling you have done are correct except H rxn < 0. H rxn <0 because in this potential energy diagram energy of the product is less thant that of reactants. Which means there is a decrease in total enthalpy of the system.
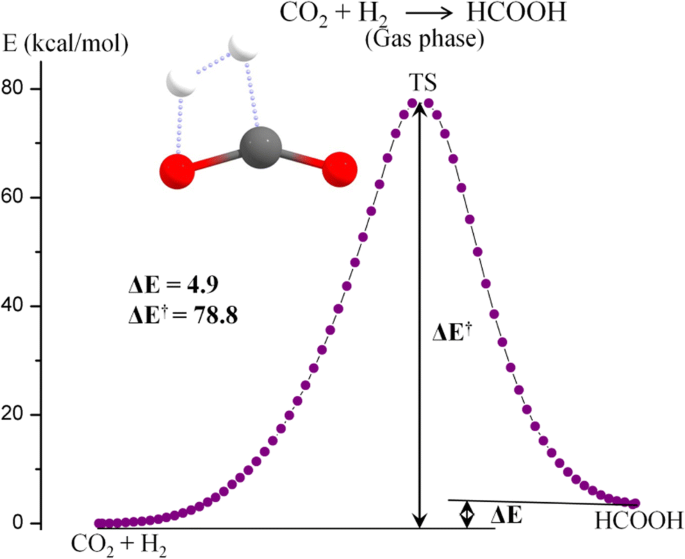
› books › chapterChapter 14, Chemical Kinetics Video Solutions ... - Numerade The following mechanism, with the accompanying energy diagram, has been suggested for this reaction: a. Based on the energy diagram, determine which step is rate limiting. b. What is the expected order of the reaction based on the proposed mechanism? c. Is the overall reaction exothermic or endothermic?
Diagram The Reaction Course Of An Exergonic Reaction Free ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Catalyzed reactions have a lower activation energy rate limiting free energy of activation than the corresponding uncatalyzed reaction resulting in a higher reaction rate at the same temperature and for the same reactant concentrations.
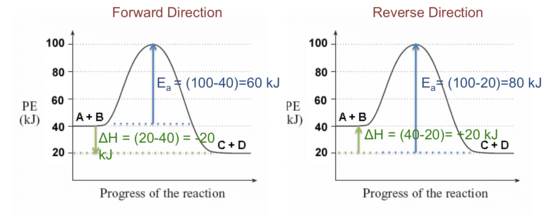
37 label the following multi-step reaction energy diagram ... Label the following multi-step reaction energy diagram. The following are steps in a reaction mechanism Step1 A_2 rightarrow A+A E_4 = 10kj delta H =-15kj Step2 A + B rightarrow C+D E_4 = 15kj delta H =-10kj Step3 C + A_2 rightarrow E+A E_4 = 25kj delta H Posted 10 months ago. Draw a potential energy diagram for a two-step endothermic reaction.
Catalysis - Chemistry 2e The reaction mechanisms, however, are clearly different. The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction mechanism with a lower activation energy.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows%(1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations.
How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
40 label the energy diagram for a two-step reaction ... Label the energy diagram for a two-step reaction. Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Label ΔH as positive or negative.
QUIZ 2 mega quizlet Flashcards | Quizlet - The type of reaction catalyzed by proteases. - An enzyme that is part of a pigment formation pathway and has a low-temperature optimum. - A protease enzyme with a low pH optimum. - The inhibitor that binds only to the ES complex and lowers the Vmax and KM. - The enzyme inhibition that can be overcome by increasing concentration of substrate.
















0 Response to "39 label the following reaction energy diagram for a catalyzed and an uncatalyzed process"
Post a Comment