38 lewis dot diagram n2
N2 Lewis Structure| Hybridization & Molecular Geometry ... N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in simplest form. Two oxygen atoms are joined by a ... Based on the Lewis electron-dot diagram that you drew, is ... Based on the Lewis electron-dot diagram that you drew, is the N2 molecule polar? Explain 2 See answers Advertisement Advertisement harshil11153 harshil11153 Answer: It has zero dipole moment. Two N atoms in nitrogen molecule have zero electronegativity difference. The bond pairs of electrons are equally distributed between two N atoms.
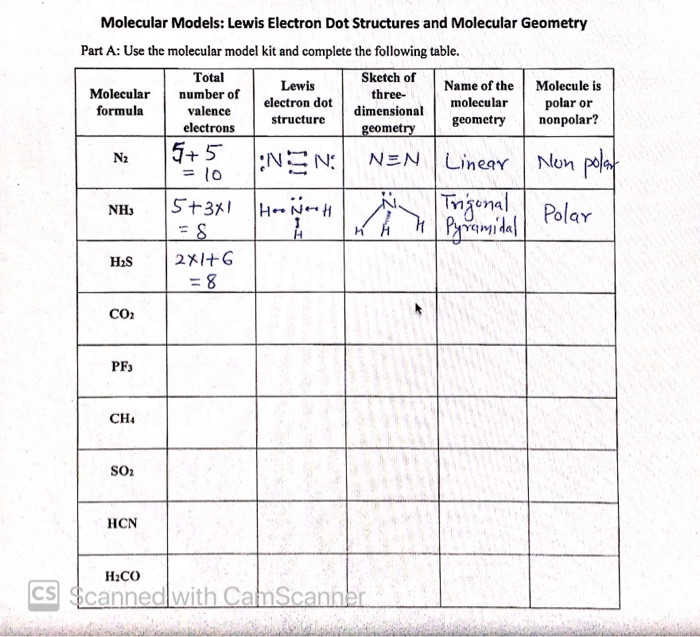
Draw electron dot structure of CO2,H2O,F2,N2 Click here👆to get an answer to your question ️ Draw electron dot structure of CO2,H2O,F2,N2. Solve Study Textbooks Guides. Join / Login >> Class 11 ... Draw electron dot structure of C O 2 ... Write the Lewis dot structure of C O molecule. Medium. View solution > View more.

Lewis dot diagram n2
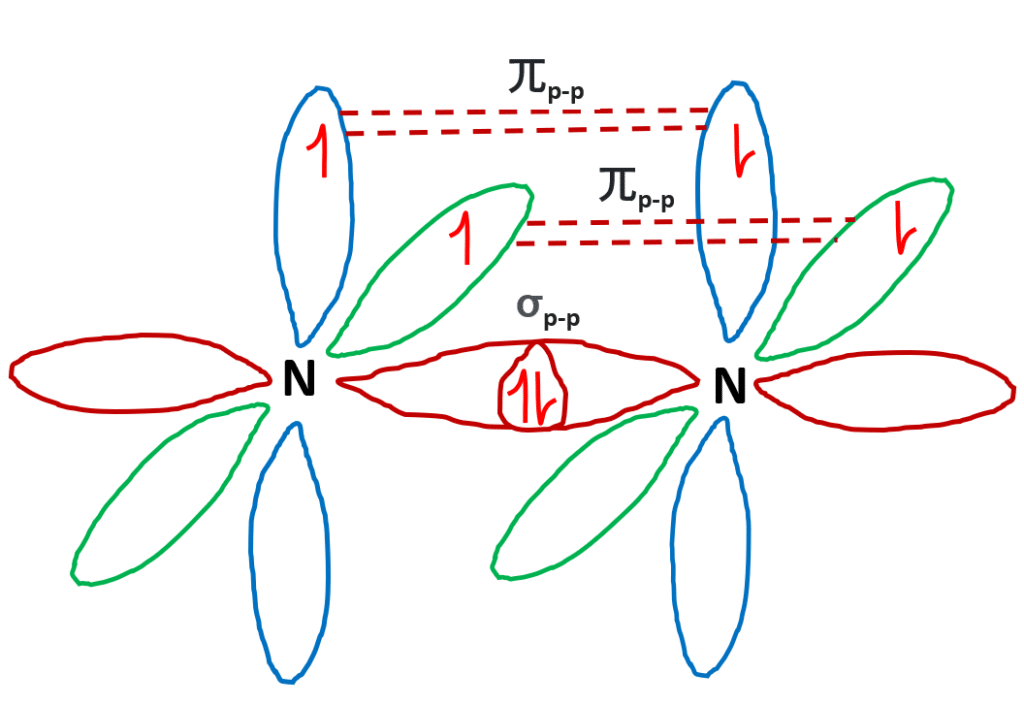
Solved 17) The Lewis electron-dot structure of N2 has ... Question: 17) The Lewis electron-dot structure of N2 has _____ nonbonding electrons pairs, bonding electron pairs,_____and a bond order of_____ This problem has been solved! See the answer See the answer See the answer done loading Nitrogen (N2) Molecule Lewis Structure Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's lewis structure. How to determine the Lewis dot diagram for N2 - Quora Originally Answered: What is the Lewis dot structure for N2? As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell.
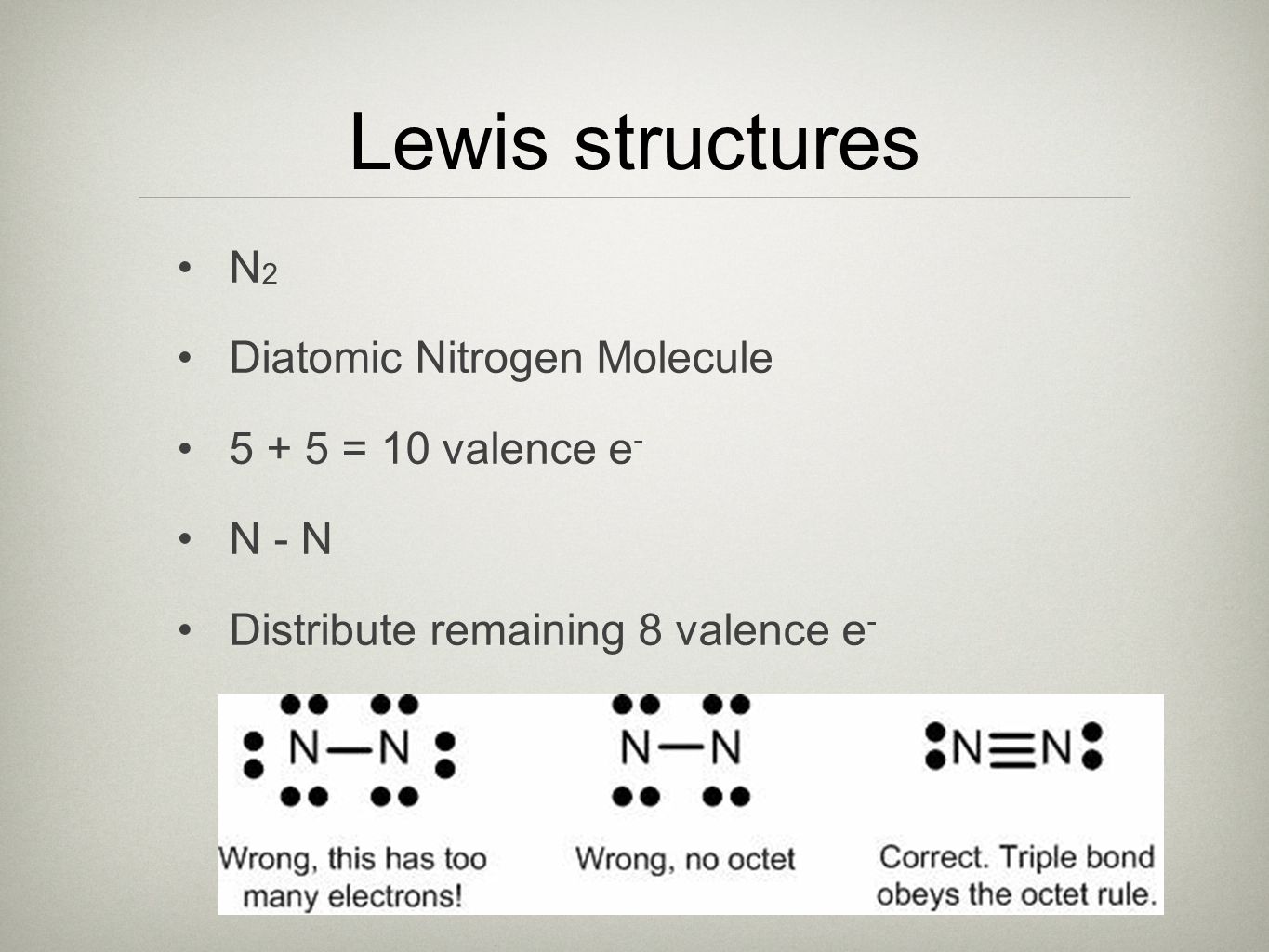
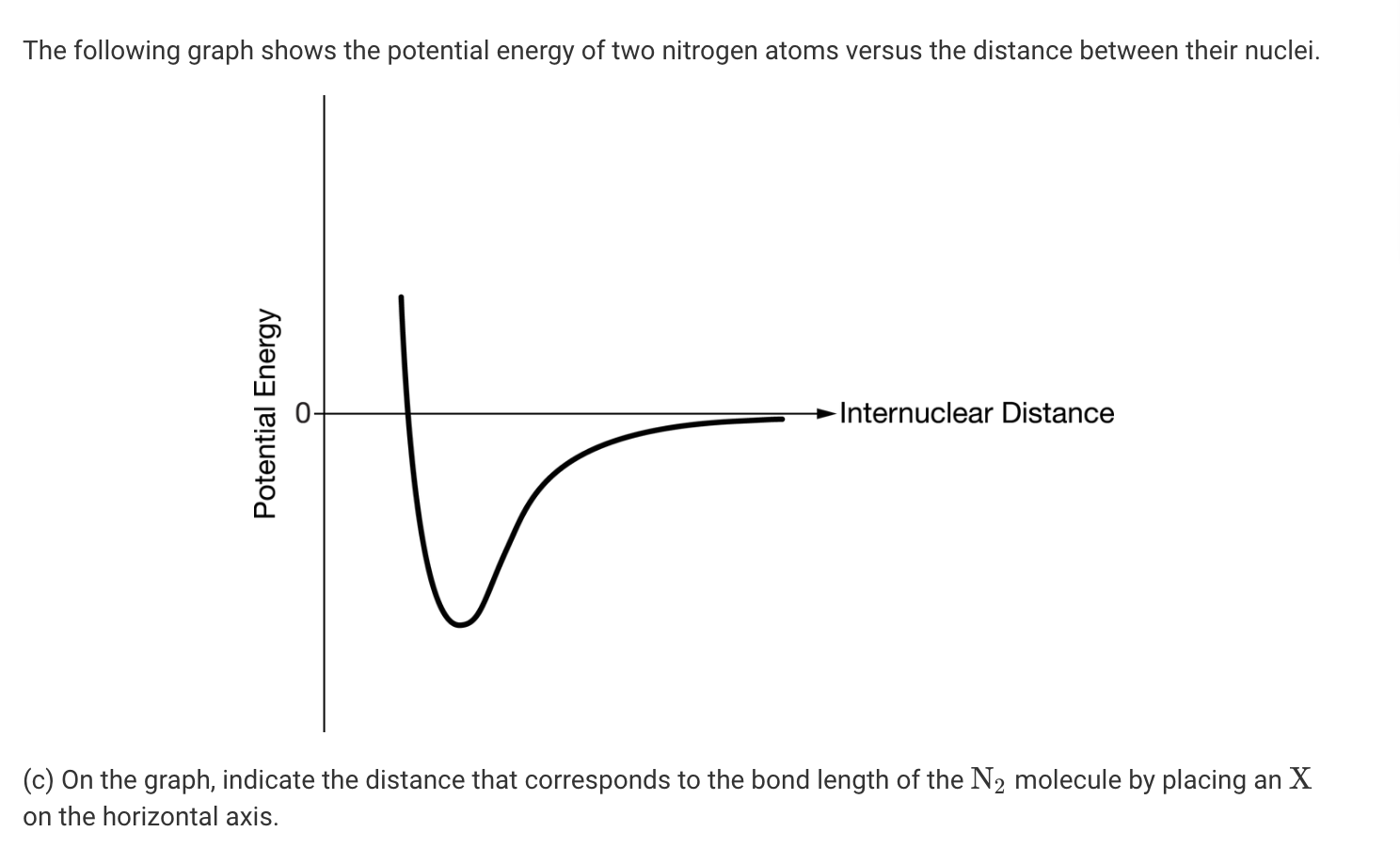
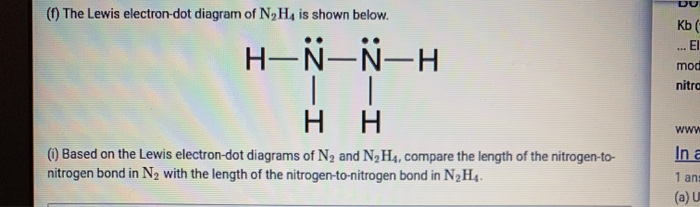
Lewis dot diagram n2. Solved Answer the following questions about N2 and N2H4 ... Answer the following questions about N2 and N2H4. (a) In the box below, draw the complete Lewis electron-dot diagram of N2. (b) Based on the Lewis electron-dot diagram that you drew, is the N2 molecule polar? Explain. (c) The following graph shows the potential energy of two nitrogen atoms versus the distance between their nuclei. What is the Lewis structure of N2? - Answers What is the Lewis dot formation of N2? The N2 molecule consists of two nitrogen atoms held together by a triple bond. Each nitrogen atom also has a lone-pair of electrons. Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below: N2 Lewis Structure, Molecular Geometry, and Hybridization Steps to Draw the Lewis structure of N2 Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
Draw Lewis dot structures of the diatomic molecules 02 and ... Oxygen gas has the molecular formula of O2 and nitrogen gas is N2. The Lewis dot structures that represent both diatomic gases are::O=O: :N:::N: N2 Lewis Structure | Lewis Structure N2 | HND Assignment How is n2 formed in n2 lewis structure? The Lewis Structure or the Lewis Dot Structure or the Lewis Dot Diagram, named after Gilbert N. Lewis, shows the diagram of the atomic bonding of the molecules or an element. It shows the lone pairs of molecules existing in a molecule. Lewis Dot Diagram For N2 - schematron.org If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like? N (triple bond)N and then two dots on each N in which ever spot is open. Share to.Lewis structure - WikipediaWhat is the Lewis structure of N2 N2 Lewis Structure: Full Guide (2022 Updated) Steps In Drawing the N2 Lewis Structure To create a Lewis structure, determine first the number of valence electrons in each atom. Nitrogen has a total of ten valence electrons—five electrons on its outermost valence shell. After determining the total number of valence electrons., connect the atoms between electron pairs.
N2 Lewis Structure: How to Draw the Dot Structure for N2 ... Drawing the Lewis Structure for N 2 Viewing Notes: Make sure you count the number of valence electrons correctly. Nitrogen is in group 5A (also called Group 15). Each Nitrogen atom has five valence electrons. Since there are two Nitrogen atoms in N 2 you have a total of ten valence electrons to work with. What is the Lewis structure of N2? | Socratic In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-. Lewis Structure of N2 (Nitrogen Gas) - YouTube How to Draw the Lewis Structure of N2 - with explanation!Check me out: Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Draw the Lewis structure for N2. Nitrogen is an unreactive ... The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
Is this diagram correct for difference between 2N and N2 One $\ce{N2}$ molecule formed: What you have drawn here is (as I have understood) Lewis dot diagram of the $\ce{N2}$ molecule. The correct way of drawing the molecule is as in 1) Two separate N atoms which have not yet formed any bond: With the concept of Lewis dot structure formula the Nitrogen atoms are represented as in 2)
Lewis Structure for N2 (Dinitrogen or Nitrogen Gas) Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
Lewis structure calculator | Lewis structure generator To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO4- 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button.
Answered: Draw the Lewis Dot Structure for N2.… | bartleby Solution for Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and…
PDF Lewis dot structure of n2 molecule Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
Answered: Draw the Lewis Dot structure for N2 (on… | bartleby Science Chemistry Q&A Library Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express your answer as a whole number. b. How many single bonds are in N2? Express your answer as a whole number.
[Expert Verified] Based on the Lewis electron-dot diagrams ... Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4. The strength of a bond is dependent on the bond length and the bond order. The higher the bond order, the shorter and stronger the bond. Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.
⚗️Based on the Lewis electron-dot diagrams of n2 and n2h4 ... College. answer. answer. answered. Based on the Lewis electron-dot diagrams of n2 and n2h4, compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. 2.
N2 Lewis Structure - Easy Hard Science The N 2 Lewis structure indicates that the N 2 molecule is perfectly symmetric. Therefore, N 2 is a nonpolar substance. Small nonpolar substances tend to be gasses. They tend to have low boiling points. For example, N 2 must be chilled to about -200 ℃ or -320 ℉ to liquify it.
How to determine the Lewis dot diagram for N2 - Quora Originally Answered: What is the Lewis dot structure for N2? As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell.
Nitrogen (N2) Molecule Lewis Structure Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's lewis structure.
Solved 17) The Lewis electron-dot structure of N2 has ... Question: 17) The Lewis electron-dot structure of N2 has _____ nonbonding electrons pairs, bonding electron pairs,_____and a bond order of_____ This problem has been solved! See the answer See the answer See the answer done loading
![Expert Answer] Draw the electron dot structure of nitrogen ...](https://hi-static.z-dn.net/files/da9/f9dadd9fdaba09b20251ec55355bd804.jpg)

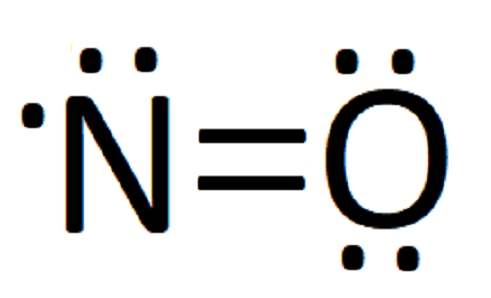
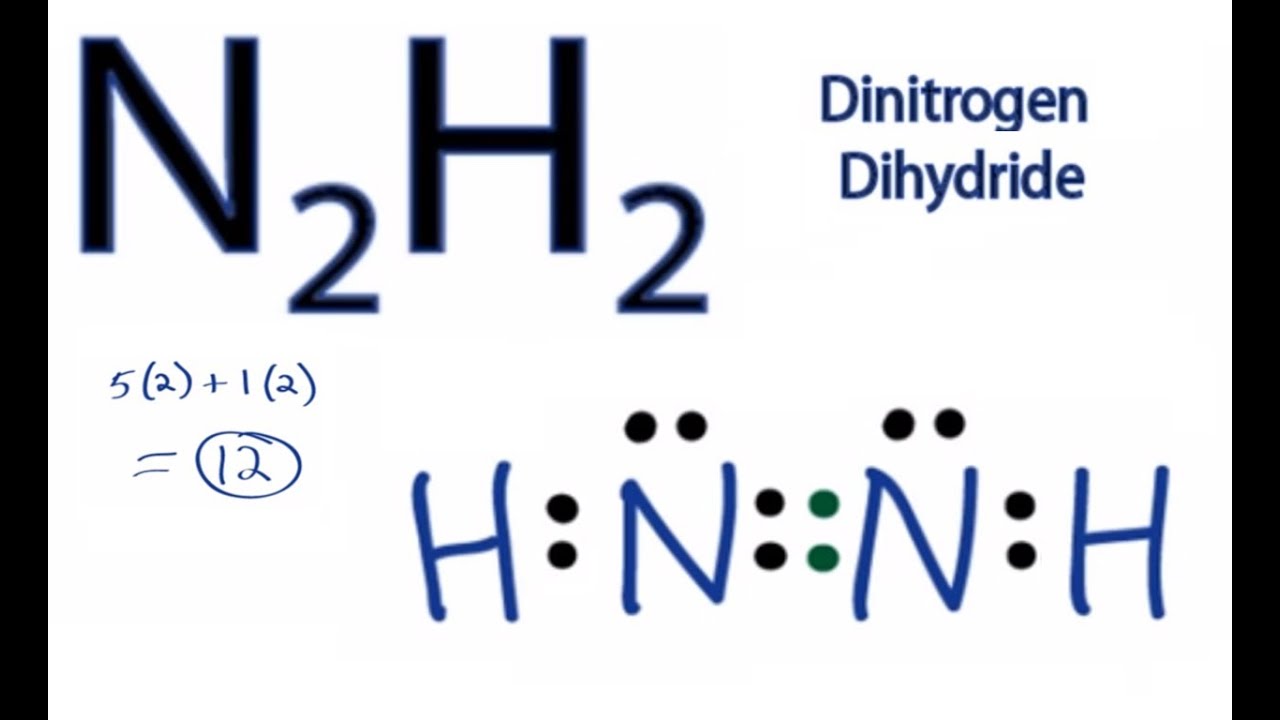




















0 Response to "38 lewis dot diagram n2"
Post a Comment