38 fluorine electron dot diagram
What intermolecular forces are present in CO_2? | Socratic 06.05.2018 · However, as shown on the diagram, the central carbon atom contains only two electron domains (two double bonds), giving the molecule a linear geometry. Dipoles from carbon-oxide bonds cancel out due to the symmetric charge distribution. In other words, carbon dioxide molecules have no net dipole/ are nonpolar hence do not engage in dipole-dipole … Electron Dot Diagram For Fluorine - Wiring Diagrams Electron Dot Diagram For Fluorine A larger outer circle has one red dot on, representing the second shell with one electron. Lithium is in Group 1 of the Periodic Table. Fluorine. Structure of a. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).
Fluorine | F2 - PubChem Fluorine | F2 | CID 24524 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...

Fluorine electron dot diagram
Lewis Dot Structure for Fluorine Atom (F) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ... Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. How to Draw the Lewis Dot Structure for NaF: Sodium ... A step-by-step explanation of how to draw the NaF Lewis Dot Structure.For NaF we have an ionic compound and we need to take that into account when we draw th...
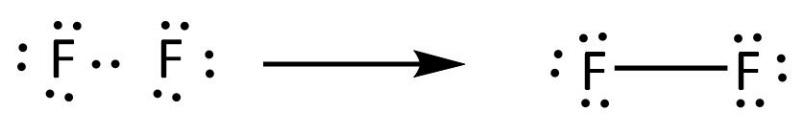
Fluorine electron dot diagram. Lewis Electron Dot Structures: Steps, Examples and Limitations 15.11.2021 · Lewis Electron Dot Structures. A covalent bond involves valence electrons of atoms interacting with each other. Gilbert Lewis proposed simplified notations taking into account the role of valence electrons. This representation involves representing the atom by its chemical symbol and valence shell electrons by dots around the atomic symbol. . Thus it gives a … How to Draw the Lewis Dot Structure for F2 : Diatomic Fluorine A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju... How to draw KF Lewis Structure? - Science Education and ... To sketch the KF Lewis structure by following these instructions: Step-1: KF Lewis dot Structure by counting valence electrons on the fluorine atom. Step-2: Lewis Structure of KF for counting valence electrons around the terminal potassium atoms. Step-3: Lewis dot Structure for KF generated from step-1 and step-2. Lewis Dot Symbols and Lewis Structures | Boundless Chemistry The former, known as a ‘Lewis dot diagram,’ indicates a pair of shared electrons between the atomic symbols, while the latter, known as a ‘Lewis structure,’ uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well. Lewis dot dragram for methane: Methane, with molecular formula CH 4, is shown. The …
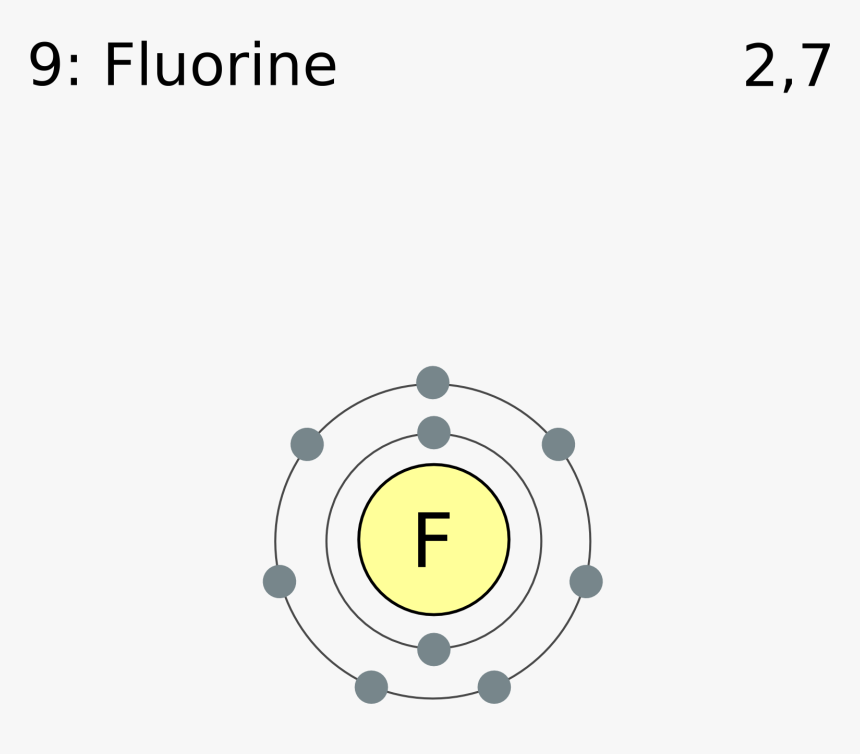
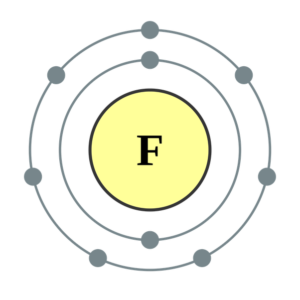
Fluorine(F) electron configuration and orbital diagram Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Fluorine Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of Fluorine atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot. The electron configuration of Fluorine Lewis structure for fluorine? - Answers The Lewis structure of fluorine contains 9 electrons, which 7 of them are valence. This means the letter F will be in the middle with 7 dots surrounded it, which would represent the 7 valence... Draw the electron dot structure of F2 (fluorine). - askIITians Draw the electron dot structure of F2 (fluorine). Our expert is working on this Class X Science answer. We will update the answer very soon.
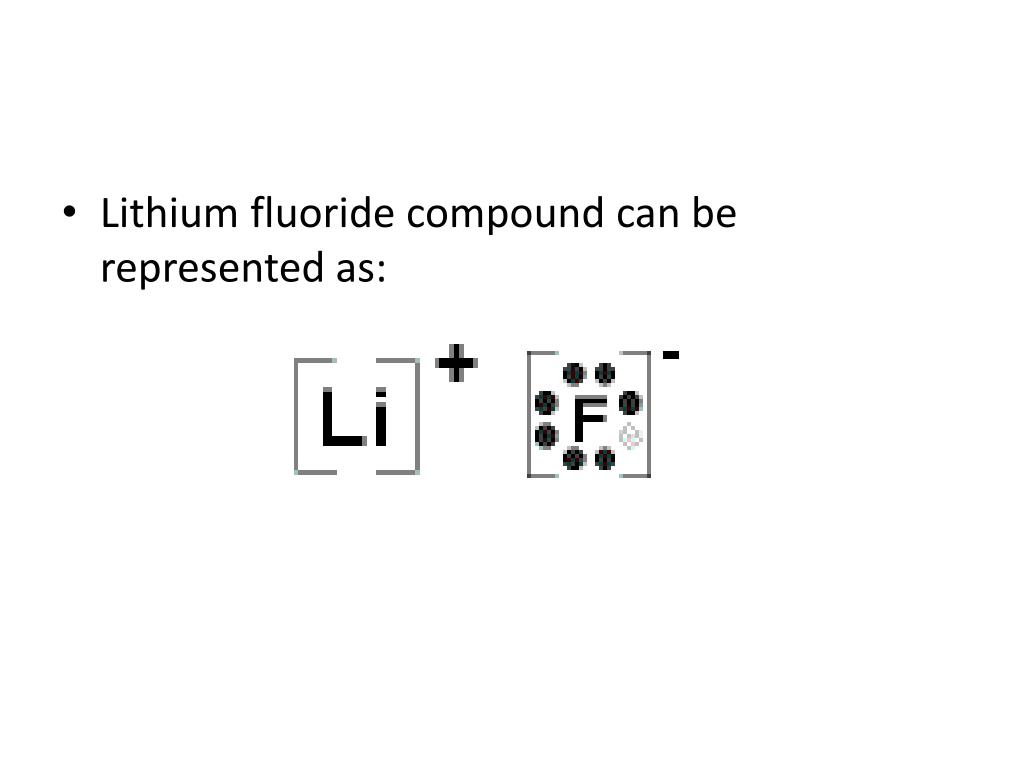
AP Chemistry 2019 Free-Response Questions - College Board The complete Lewis electron-dot. diagram for the urea molecule is shown above. (a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule. (b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and four water molecules are represented in the box below. Draw ONE dashed line (----) to indicate a possible ... Lewis Electron Dot Diagram For Fluoride Ion In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF. Lithium atom loses one electron to form the cation Li+. Octet rule - Wikipedia The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. Lewis Dot Diagram For Fluorine Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Electron Dot Diagram For Fluorine - schematron.org Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron.
Diagram - Fluorine Fluorine's Diagram. ... Mass Number: 18.9984032 Electron configuration: 1s2,2s2,2p5 or [HE] 2s2 2p5 Valence Electrons: 7 Orbital Notation: Electron Dot Notation: Powered by Create your own unique website with customizable templates. Get Started ...
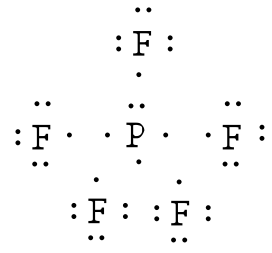
Phosphorus trifluoride (PF3) lewis dot structure ... Lewis dot structure for PF3. As you see in this PF3 lewis dot structure, phosphorous and fluorine completed their octet, and everything looks fine, but, for the sake of satisfaction, we should also determine the formal charge in the above structure to know whether it is stable or not. 6. Check the stability with the help of a formal charge concept
filestore.aqa.org.uk › sample-papers-and-markCOMBINED SCIENCE: TRILOGY - AQA Write about electron transfer in your answer. [4 marks] 6 ... Figure 4 represents one molecule of fluorine. Complete the dot and cross diagram ... A fluorine atom can ...
› science › chemistryTips for Identifying Intermolecular Forces - Concept ... Hydrogen bonding is actually not a bond, remember it’s an intermolecular force. This type of intermolecular bond is the strongest. Dipole – Dipole in the middle. So hydrogen bonding is like the name says. It involves hydrogen, but it only involves 3 elements, F Fluorine, O Oxygen and Nitrogen, N.
How many dots would be placed around fluorine in a Lewis ... 7 dots would be placed around fluorine in a Lewis electron dot diagram. Some features of Lewis dot diagram are, It shows localization of valence electrons. They are two dimensional structures. It doesn't show shape of the molecule. Thus, we can conclude that, 7 electrons are present in the valence shell of fluorine.
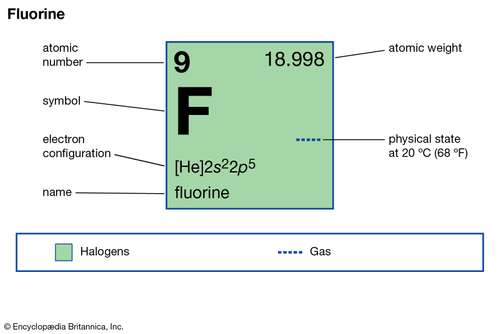
Electron Configuration for Fluorine (F) - UMD Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital.
How to Draw the Lewis Dot Structure for KF: Potassium ... A step-by-step explanation of how to draw the KF Lewis Dot Structure.For KF we have an ionic compound and we need to take that into account when we draw the ...
Which Lewis Dot Diagram Represents A Fluoride Ion Draw a Lewis electron dot diagram for an atom or a monatomic ion. a simple way of representing those valence electrons would be useful. Fluoride-Neon. 31 Which electron shell contains the valence electrons of a radium atom in the ground state? 35 Which Lewis electron dot diagram represents a. fluoride ion.
Rutherford Scattering - Atomic Nuclei | Atomic Structure ... How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
PDF Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
Lewis Dot Diagram For Fluorine - schematron.org In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the . Fluorine is in Group 17 of the Periodic Table.. And thus the neutral atom has 7 valence electrons.
Create a electron-dot diagram bond between two atoms of ... create a electron-dot diagram for bond between two atoms of fluorine. Wiki User. ... Carbon and fluorine can combine to form carbon tetrafluoride, which has the formula CF4. Many of these ...
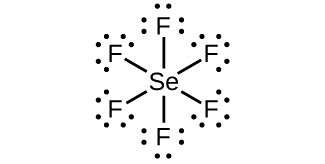
Selenium tetrafluoride (SeF4) lewis dot structure ... First of all, determine the valence electron that is available for drawing the lewis structure of SeF4 because the lewis diagram is all about the representation of valence electrons on atoms. So, an easy way to find the valence electron of atoms in the SeF4 molecule is, just to look at the periodic group of selenium and fluorine atoms.
MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
How to Draw the Lewis Dot Structure for NaF: Sodium ... A step-by-step explanation of how to draw the NaF Lewis Dot Structure.For NaF we have an ionic compound and we need to take that into account when we draw th...
Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Lewis Dot Structure for Fluorine Atom (F) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ...

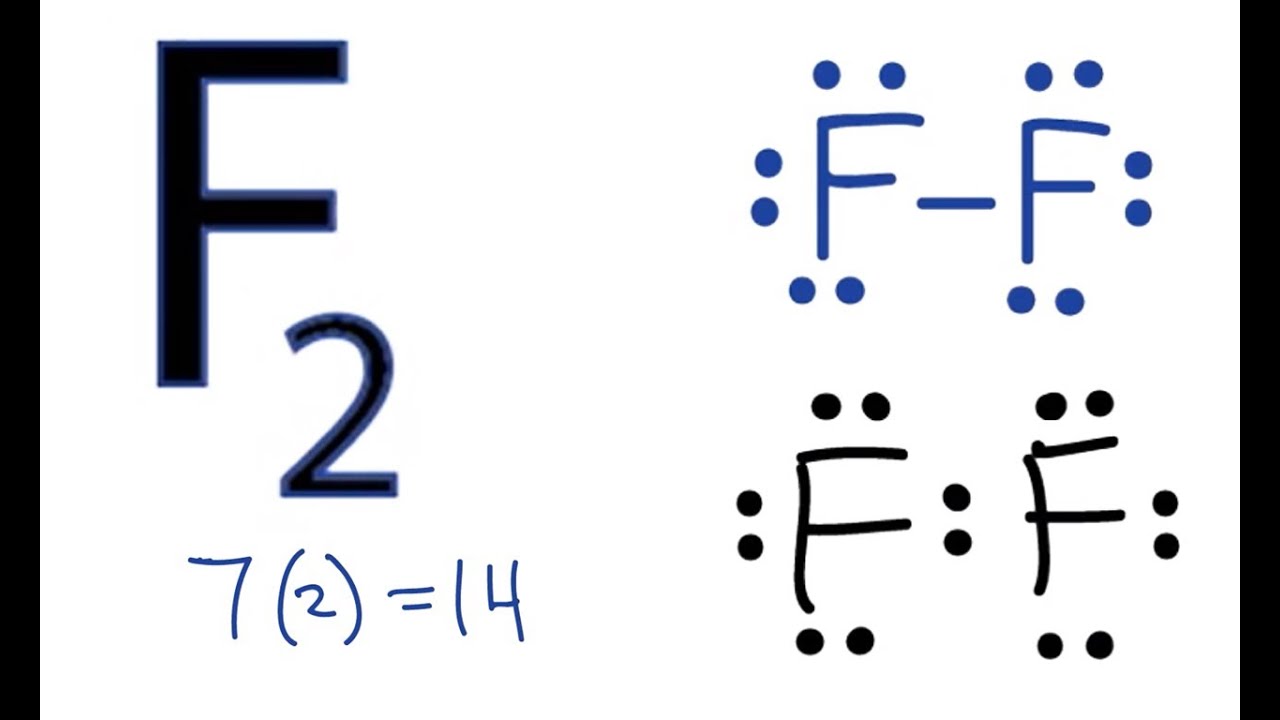









![Expert Answer] draw the electron dot structure of F2 - Brainly.in](https://hi-static.z-dn.net/files/df8/67df4fdbd3d368c6287c82f3548774aa.png)
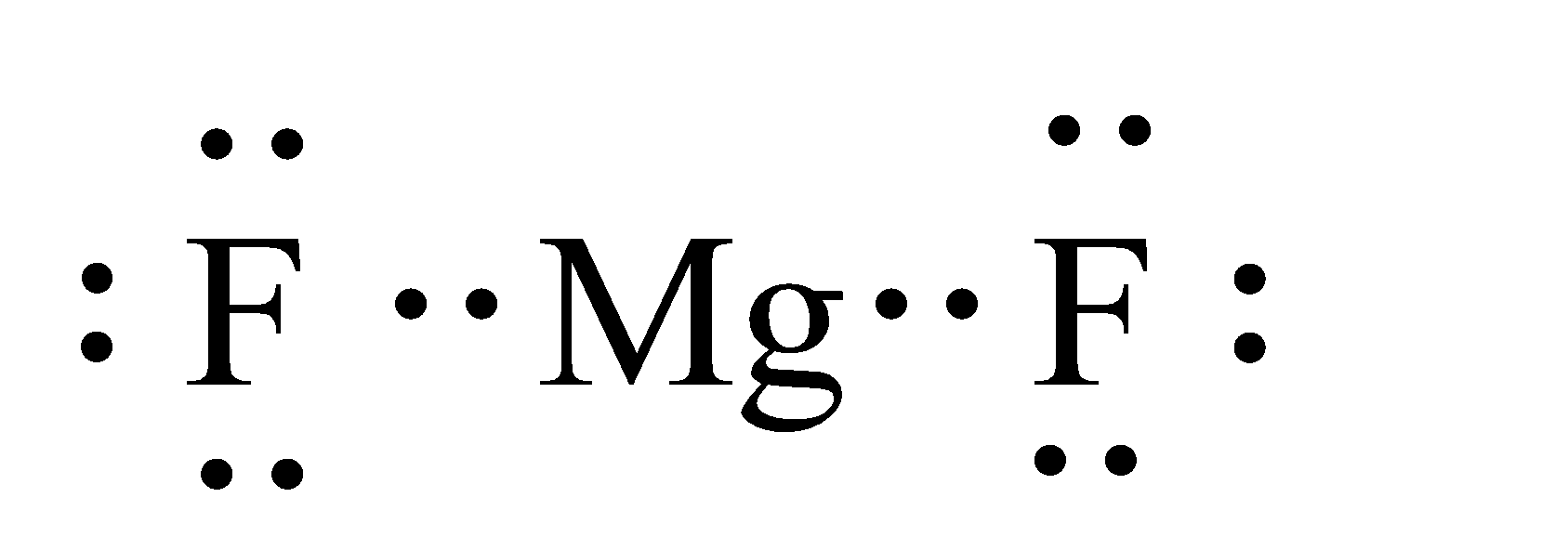






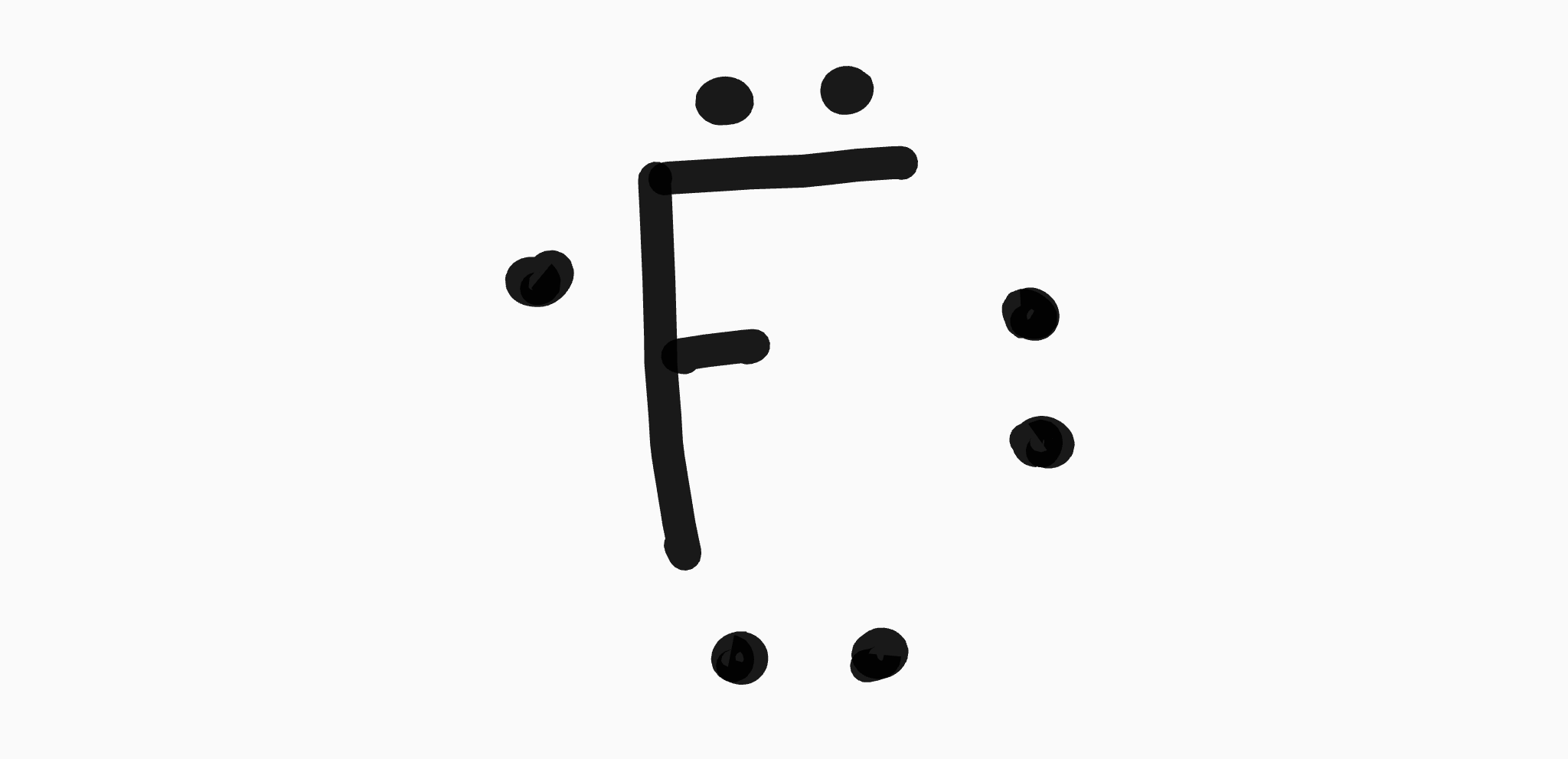

0 Response to "38 fluorine electron dot diagram"
Post a Comment