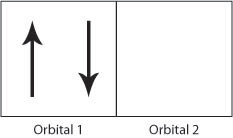
36 which diagram shows a pair of electrons that have opposite spins?
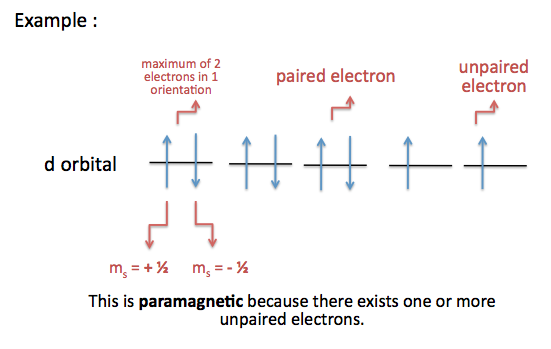
Distributing 8 electrons over 6 molecular orbitals leaves the final two electrons as a degenerate pair in the 2pπ* antibonding orbitals resulting in a bond order of 2. As in diboron, these two unpaired electrons have the same spin in the ground state, which is a paramagnetic diradical triplet oxygen.
This interview was done by that balloon boy's father, Richard Heene, back in 2004. Dr. Podkletnov is currently working on classified research programs in Russia. Lots of interesting information here on anti-gravity propulsion and potential energy sources. The transcript is very long, so it's split into five posts. --- --- **Host:** Personally speaking, for me. In all honesty, you're like my hero, because since I was 13 years old, I've been fascinated with gravity. I told my father that one day...
**Brushless DC motors have some significant advantages over their competitors, such as brushed motors, largely because of the electronic commutation. It allows the controller to switch the current promptly and thus regulate the motor’s characteristics effectively. In this article, we’ll consider the peculiarities of a brushless DC motor controller. You will learn about its operating principles as well as the design features and challenges you should know about before building your own device.** ...

Which diagram shows a pair of electrons that have opposite spins?
According to Pauli's exclusion principle, one atomic orbital can have a maximum of two electrons with opposite spins. The same is also true for molecular orbitals. The pairing of electrons in the degenerate molecular orbitals will take place only when all the degenerate molecular orbitals are singly occupied.
Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron.
If two electrons are paired, then they will have an opposite spin from each other. In terms of comparing non-paired electron spin, we can refer to each electron's ms value to see if electrons have the same or opposite spin. Electrons in a sub-shell will all have the same spin until electron pairing occurs within that given sub-shell. Top Michael 3F
Which diagram shows a pair of electrons that have opposite spins?.
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain two electrons (electron pair) with opposite spins. How many unpaired electrons does B have?
Which diagram shows a pair of electrons that have opposite spins? O1 1 个个 - DocumenTV. Which diagram shows a pair of electrons that have opposite spins? O1 1 个个. Which diagram shows a pair of electrons that have opposite spins?
Over the month of December I posted two songs per day in the Daily Discussion threads (one single and one album track (mostly)) that I felt went unnoticed by most people but would potentially be enjoyed, and this is a compilation of those write-ups. There’s a definite pop focus but I picked these tracks with an ear for diversity of sound/artistry so there’s quite a few different things going on here and some picks that might make you roll your eyes, but I think there’s something for everyone! ...
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
Inspiring orchestral music begins as a scene from one of the galaxy's millions of ecumenopolises fades in. Kilometer tall metal towers reflect the first rays of dawn as residents tend to their balcony gardens. A contralto voice narrates. "How blessed we are that so few people in the Galactic Commonwealth lack life's necessities. We wake in warm beds, eat to our content from auto fridges, and live out happy lives in any one of 50 billion star systems. But there are those left in the cold with l...
As shown, the 1s subshell can hold only two electrons and, when filled, the electrons have opposite spins. Hund's Rule When assigning electrons in orbitals, each electron will first fill all the orbitals with similar energy (also referred to as degenerate) before pairing with another electron in a half-filled orbital.
Nov 06, 2021 · Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy. We then fill the orbitals with the required number of valence electrons according to the Pauli principle. This means that each molecular orbital can accommodate a maximum of two electrons with opposite spins.
The "Atomic Hotel" has a long standing tradition since 1925 to also follow the Pauli Exclusion Principle, where electrons can occupy the same room (energy level), only if the electrons have opposite spins as not to interfere with another electron guest.
This is the edited version of Fresh Blood. The book is available on [Amazon/KU](https://geni.us/ztNL). For those of you who don't want to read the full version, I'll post a brief synopsis after the chapters I am allowed to post, which should allow you to continue on with the chapters of book 2 (starting up next week). --- Power loss. There were a lot of ways to meet one's end in space. Power loss, if extended, could be just as sure as any of them. Lack of power meant not just no lighting, ...
As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins. What configurations violate Pauli exclusion principle? The 1s and 2s subshells for beryllium atoms can hold only two electrons, and when filled, the electrons must have opposite spins or have the same four quantum numbers.
Chemistry. High School. answer. answered. Which diagram shows a pair of electrons that have opposite spins? 011. 1. 011. o 11h.
Oxygen atomic number 8 has a pair of electrons in any one of the 2 p orbitals the electrons have opposite spins and a single electron in. Electronic configurations describe each electron as moving independently in an orbital in an average field created by all other orbitals.
Chlorine follows the octet rule but iodine shows an expanded octet due to the presence of d electrons in the Lewis structure of ICl3. The geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape. ICl3 has three bond pairs and two lone pairs of electrons.
The bond order of H2 is 1 which shows that the hydrogen has only one bond. The BMO contains 2 electrons with opposite spins and ABMO is empty. if the number of electrons in bonding orbitals is greater than the antibonding orbital then the molecule is stable. Thus, H2 is a stable molecule.
The use of the above diagram of the virtual particle producing a quark–antiquark pair was featured in the television sit-com The Big Bang Theory, in the episode "The Bat Jar Conjecture". PhD Comics of January 11, 2012, shows Feynman diagrams that visualize and describe quantum academic interactions , i.e. the paths followed by Ph.D. students ...
Which of the following is the correct orbital diagram for a nitrogen (n) atom_
The orbital diagram for nitrogen shows how electrons fill the sub-orbitals. ... Sub-shells are filled with pairs of electrons with opposite spins that share the same orbital path around the ...
The correct answer on edgenuit.y is A) updown the arrows that are combined together. Im sorry im late but I hope this helps anyone else . The answer will be a The pair with opposite spins is up down The spins of electrons are designated using arrows and opposite spins are designated by arrows facing in the opposite directions.
The electrons of a shared pair must have opposite spins. ... The MO diagram shows the relative energy and number of electrons in each MO. ... A species with more electrons in bonding than antibonding molecular orbitals will have a positive molecular orbital bond order.
According to the Aufbau principle, the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level, and proceeding to the highest, with each orbital holding a maximum of two paired electrons (opposite spins). Electron shell #1 has the lowest energy and its s-orbital is the first to be filled.
In the best understood superconductors, it is phonons that bind electrons into pairs. In these superconductors, the partnered electrons are required to have opposite values of their spin —a quantum property related to how quantum particles orient themselves in a magnetic field.
The illustration shows how the spins must be opposite for the hydrogen 1s atomic orbitals to ... the overlap of atomic orbitals having single electrons with opposite spins. ... and Diagrams 4:35
This is the 874th online community I've tried. Hopefully, this will be the last. Terry told me about this place, told me you guys have experience with… weird stuff. The Chewy-Man isn't the only weird thing out there I'm sure. I know from the Bigfoot and UFO forums that every inexplicable or paranormal phenomenon has its storm chasers. I've got my fingers crossed that here this post finds someone, anyone, who might be able to shed some light on just what the heck is going on in my small town. ...
For two electrons to occupy the same orbital, they need to have opposite spins. This means that a pair of electrons in the ground electronic state will have opposite spins. These electrons occupy the ground singlet state. When molecules are exposed to radiation, these electrons can get "excited" to higher energy levels.
High School. answer. answered. Which diagram shows a pair of electrons that have opposite spins? O1. 1. 个个. 1. See answer.
Electrons in higher energy levels (e.g. higher n numbers) have a wider range of l values to choose from. L can be any integer number between n‐1 and 0. For example, an electron in n = 4 could have an l value of 3, 2, 1 or 0. But there is a better way to think about l.
As another example, oxygen has 8 electrons. The electron configuration can be written as 1s 2 2s 2 2p 4. The orbital diagram is drawn as follows: the first 2 electrons will pair up in the 1s orbital; the next 2 electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals.
Two electrons of opposite spin (necessary to complete the dupletof the hydrogen atom) are attracted to the proton and this attractive potential pulls them together to yield an electron pair localised to the internuclear region. This is illustrated in the LDQ structure of hydrogen fluorideshown below. The LDQ structure of hydrogen fluoride.
SHOW ANSWER The pair with opposite spins is up down The spins of electrons are designated using arrows and opposite spins are designated by arrows facing in the opposite directions. Electrons must occupy opposite spins when they are in the same orbital in order to ensure that they are in their most stable configuration. Search for other answers
It has integral (or half-integral) values of +½ and -½. +½ designates the up spin and -½ shows the down spin. According to Pauli's exclusion principle, no two electrons in the same atom can have the values of all quantum numbers identical. It means two electrons in the same orbitals must have opposite spins.
Hund's rule states that electrons do not pair up in orbitals until they have to; for example in a 2p subshell there are 6 possible electrons (2 in each orbital). If there are 3 electrons in the said 2p subshell, they will each occupy an orbital rather than being in the same one.
*The electrons of a shared pair must have opposite spins *a covalent bond is formed by the overlap of an orbital from each of the bonding atoms According to valence bond theaory, a(n) ________ bond is formed by the overlap of orbitals from two atoms.
But the team's theory suggests that in graphene this traditional pairing mechanism can not only pair electrons with opposite spins but also pair electrons with the same spin.
At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Because all the 2p orbitals are degenerate, it doesn't matter which one has the pair of electrons.
Answer: The Pauli exclusion principle says that two electrons can occupy the same orbital only if the electrons have opposite spins. Explain that an oxygen atom contains eight electrons. The orbital of lowest energy, 1s, has one electron, then a second electron of opposite spin.
*Continuing* I wrap the six road flares, now spray-painted brick-red and stickered with the appropriate manufacturer's labels, with black electrician’s tape into a hexagonal cross-section, closest-fit bundle. I have a black plastic project box that contains a battery for ‘long-lasting power’ or so the manufacturer claims. An Arduino board that I programmed the other night that runs the wee little speaker and set of blinking LEDs I had mounted on the box. From the box sprout a pair of tightly c...
: fifth diagram shows the correct configuration.as helium only has 2 electrons and therefore it possesses a configuration of 1s2. because the 1s orbital is full with 2 electrons and any additional electrons will have to go in a new energy level.














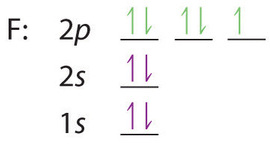










0 Response to "36 which diagram shows a pair of electrons that have opposite spins?"
Post a Comment