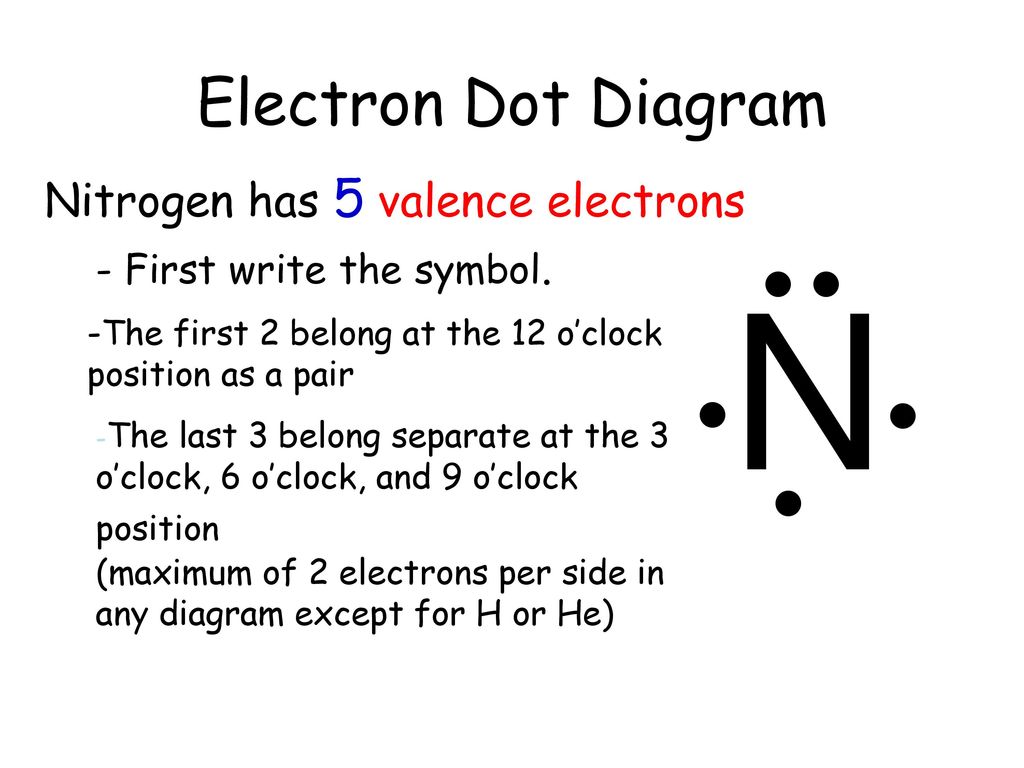
36 lewis dot diagram nitrogen
Lewis Dot Diagram Shows on the valence electrons (outermost) surrounding the chemical symbol He Na Draw a Lewis Dot Diagram for nitrogen in your notes! PERIODIC TABLE, VALENCE ELECTRONS & LEWIS DOT DIAGRAMS Columns (groups) tell us how many valence electrons! PERIODIC TABLE, VALENCE
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ...
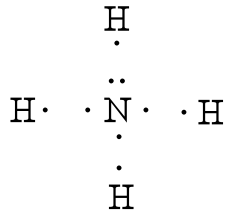
Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.

Lewis dot diagram nitrogen
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
I quickly take you through how to draw the Lewis Structure of NO2 (Nitrogen Dioxide) . I also go over hybridization, shape and bond angles.
Lewis dot diagram nitrogen.
Note: The most important thing about the Lewis dot structure is that only valence electrons take part in chemical bonding. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2.
NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.Each fluorine atom has three lone pairs. Steps of drawing lewis structure of NF 3. You have to follow few steps to draw the lewis structure of NF 3.Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps ...
Drawing the Lewis Structure for NF 3. Viewing Notes: NF 3 (Nitrogen trifluoride) is very similar to the NCl 3 and NH 3 Lewis structure.; In the NF 3 Lewis structure (and all structures) hydrogen goes on the outside.; Hydrogen only needs two valence electrons to have a full outer shell. In the Lewis structure for NF 3 there are a total of 8 valence electrons.
Lewis Electron Dot Structure for the molecule: CO 2. An oxygen atom has 6 valence electrons and a carbon atom has 4. So carbon shares 2 with one oxygen atom and 2 with the other oxygen atom. Hence two double bonds are formed. Lewis Electron Dot Structure of the molecule: CO (carbon monoxide) The carbon atom has a valency of 4.
For "dinitrogen gas", :N-=N:. For atomic nitrogen, Z=7. There are thus 7 electrons to distribute per nitrogen atom, 2 of which are inner core, and these may be ignored. So we distribute 5 electrons around EACH nitrogen centre and come up with a triple bond. This Lewis structure reflects the shortness and strength of the bond in the dinitrogen molecule, which are experimental findings.
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
A step-by-step explanation of how to draw the N3- Lewis Dot Structure.For the N3- Lewis structure use the periodic table to find the total number of valence ...
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
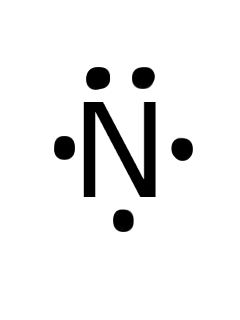
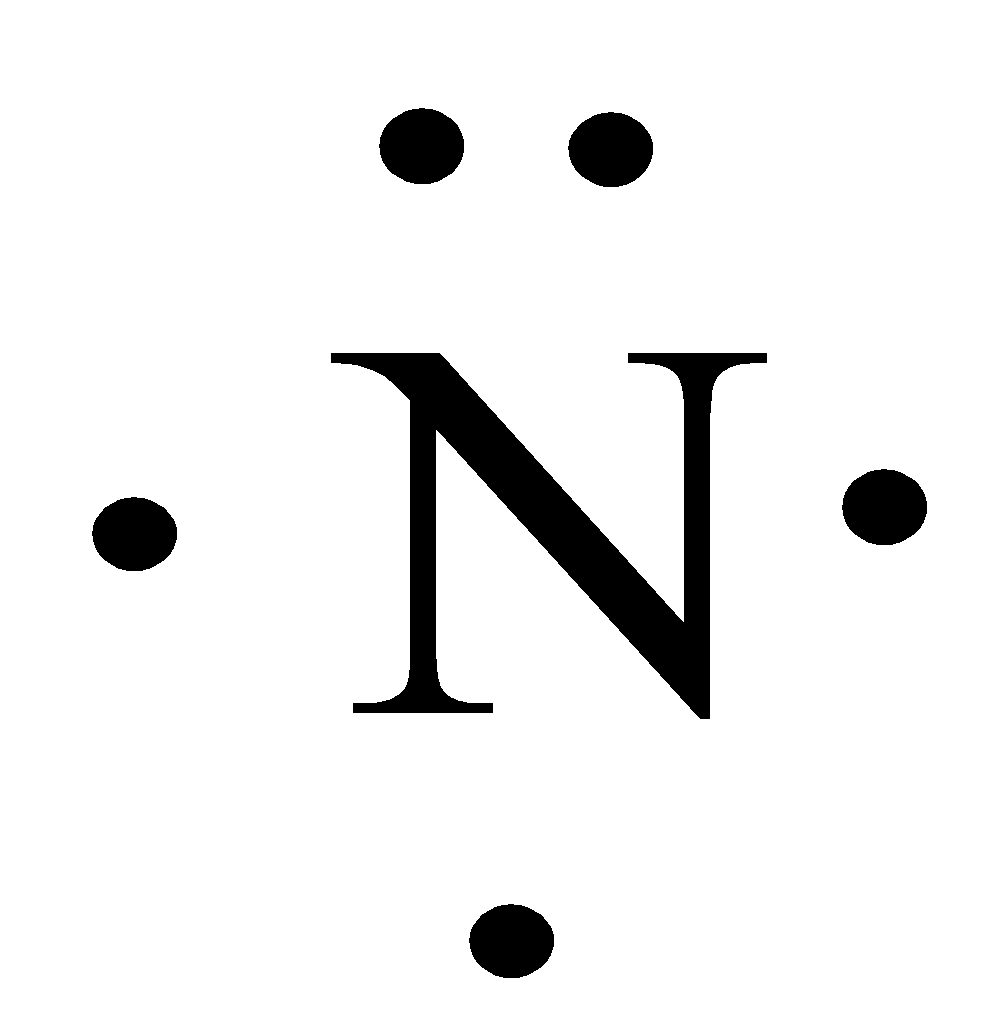
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
A step-by-step explanation of how to draw the NBr3 Lewis Dot Structure (Nitrogen tribromide).For the NBr3 structure use the periodic table to find the total ...
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.



![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://d1hj4to4g9ba46.cloudfront.net/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)












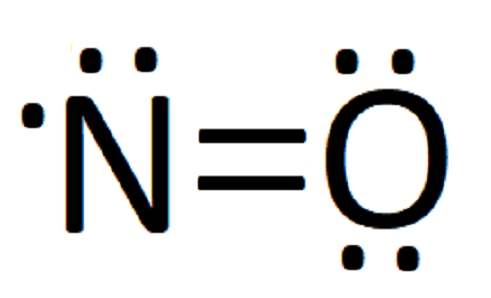



![Expert Answer] electron dot structure of Nitrogen molecule ...](https://hi-static.z-dn.net/files/d68/c596d1dd5842287e6938ce11c21acb41.png)





0 Response to "36 lewis dot diagram nitrogen"
Post a Comment