38 nacl lewis dot diagram
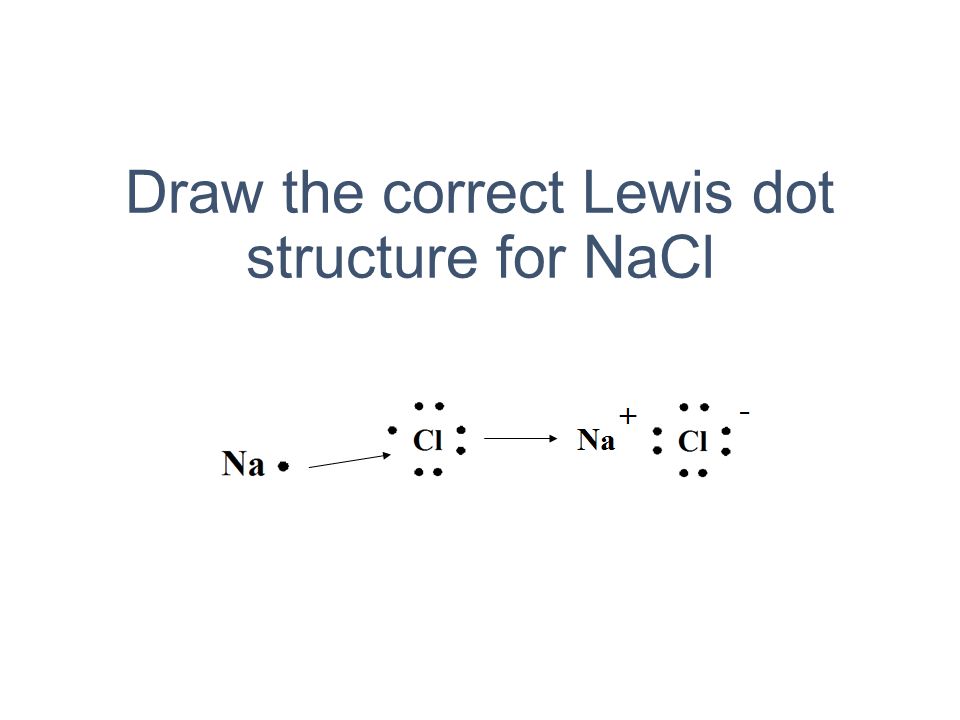
The lewis dot structure of nacl consists of a chloride ion surrounded by eight electron dots four pairs and a sodium ion bonded to that chlorine ion. Although these electron arrangement diagrams show how the ionic bond is formed and the electronic structure and electrical charge on the ions they do not give any idea on the relative size of the ... Draw The Correct Lewis Dot Structure For Nacl Ppt Video Online Download . You can easily learn chemistry. How to type lewis dot structures in word. Users can use the circle tool to create the circles representing electrons and the line tool to create the lines indicating chemical bonds. Our cartoon picture of a molecule is limited but can give ...
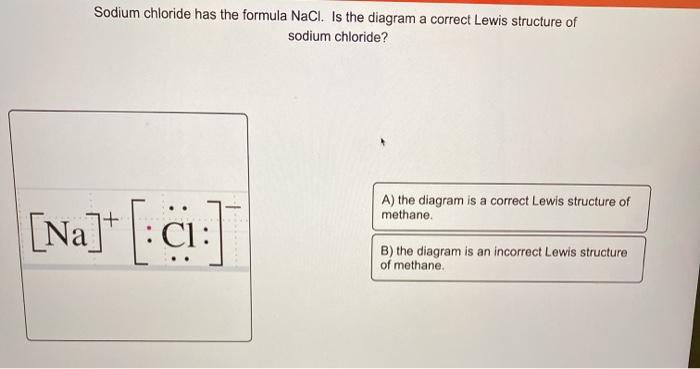
NaCl lewis structure is unique and very interesting to draw because it is an ionic compound formed from metal(Na+) and nonmetal(Cl-). The lewis dot structure of NaCl contains one positive charge on sodium metal and one negative charge on chlorine nonmetal.

Nacl lewis dot diagram
What is the electron dot structure for NaCl? Sodium atom will loose one electron to gain noble gas configuration and form sodium cation with +1 charge. Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge. In sodium chloride the one electron from sodium metal gets transferred to chlorine atom. 3.2 NaCl 3.3 AlCl 3, 3.4 PCl 3, 3.5 CaO 3.6 F 2 Question 4 Every day we use table salt to season our food. Table salt is the common name for sodium chloride. 4.1 Give the chemical formula for sodium chloride 4.2 Draw Lewis dot diagrams for the sodium ion (Na+) 4.3 Draw Lewis dot diagrams for the chlorine ion (Cl-) The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
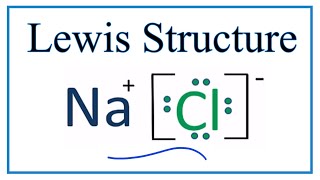
Nacl lewis dot diagram. 3 Sept 2019 — where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges ... Thus, we write the lewis structure for nacl as: The lewis structure is drawn in such a way that the octet of each atom is complete. Nacl Lewis Structure - PPT - Ionic Compound Properties, Lewis Dot Structures & Polyatomics PowerPoint Presentation - ID : The left diagram shows a lewis dot structure of chlorine with .. Thus, sodium losses one ... A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc... The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure.For the NaCl Lewis structure, calculate the total number of valence electrons for the ... The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Thus, to determine the oxidation number of the two atoms, we count the electrons It is important to keep the formal charges as low as possible. and hence several thousands times slower than electrons). Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. 2. Table 4.5. 2: Lewis Dot Symbols for the Elements in Period 2. Ionic compounds are produced when a metal bonds with a nonmetal. Stability is achieved for both atoms once the transfer of electrons has occurred. The image below shows how sodium and chlorine bond to form the compound sodium chloride.
Lewis Dot Structure for Francium For example, an element in group 1A will have one valence electron, and therefore, one dot. An element in group 2A will have two valence electrons, and (you ... Sodium Electron Dot Diagram 73 Lewis Symbols And Structures Chemistry. Sodium Electron Dot Diagram Exceptions To The Octet Rule. Sodium Electron Dot Diagram Ionic Bonding Ws 1. Sodium Electron Dot Diagram Dot Diagram For Cyanide Wiring Diagram Directory. Igcse Chemistry 2017 140 Draw Dot And Cross Diagrams To. Lewis Dot Diagram For Nacl Diagram Resource Gallery. Diagram To Show Ionic Bonding In Sodium Chloride Stock 21 Oct 2014 — The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet.
table salt, NaCl. Procedure for a Covalent Compound: 1. Draw the Lewis dot structure for each atom of the molecule to show how many valence electrons are present in each atom of the molecule. For example, the carbon atom in CO 2 in carbon dioxide has four valence electrons, and the oxygen atoms have six valence electrons. 2.
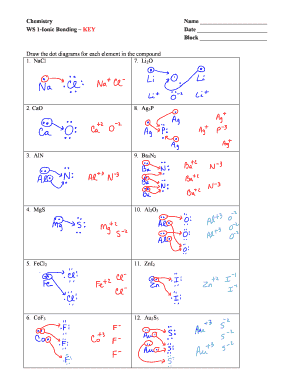
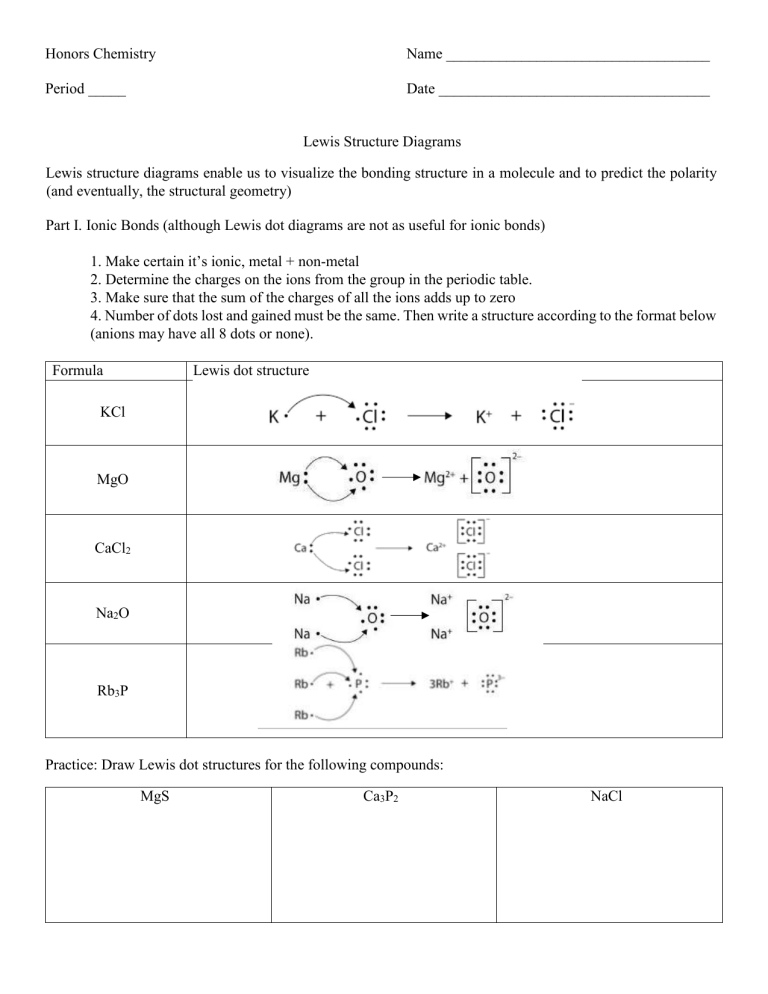
Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1. The Lewis dot structure of ionic bonds such as NaCl is formed by looking at both ...
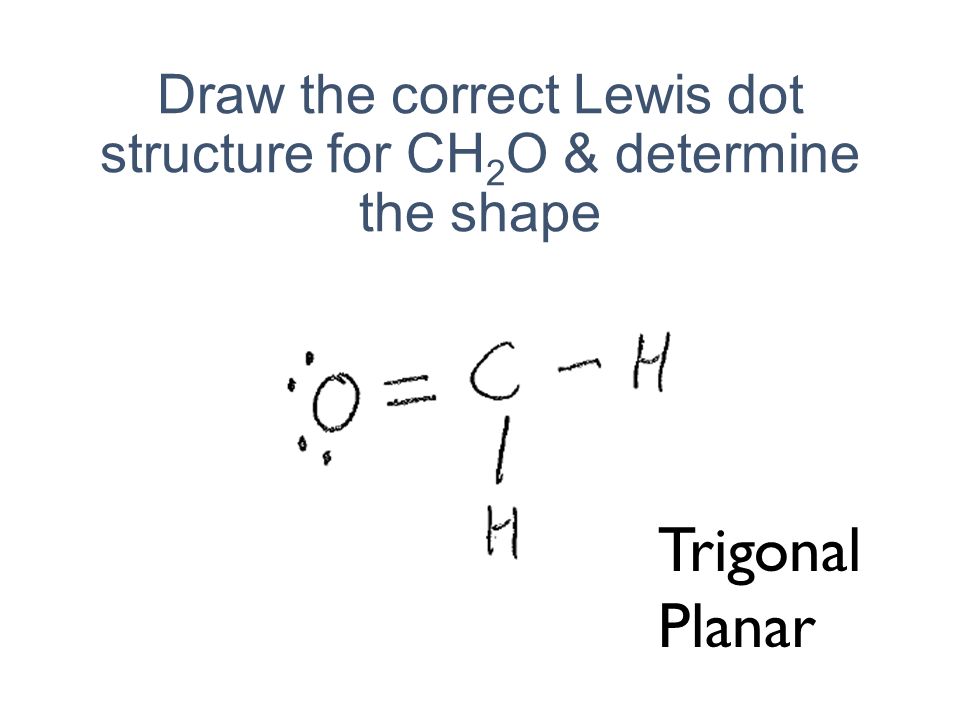
Chemists often depict a bond with a line, so sodium chloride can be written as Na -Cl. Draw the correct Lewis dot structure for CH2O & determine the shape Trigonal Planar. A sodium ion (2,8)+. Diagram of bonding in sodium chloride. A sodium atom gives an electron to a chlorine atom. The result is a sodium ion (2,8)+ and a chloride.
The lewis dot structure of nacl consists of a chloride ion surrounded by eight electron dots four pairs and a sodium ion bonded to that chlorine ion. In the case of the sodium cation the filled shell is the outermost of the core electron shells.
We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +. H. H. H H.. or H2. H. Cl. H Cl. + or HCl. 2. HCl molecule . The left diagram shows a Lewis dot structure of sodium with .. ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.
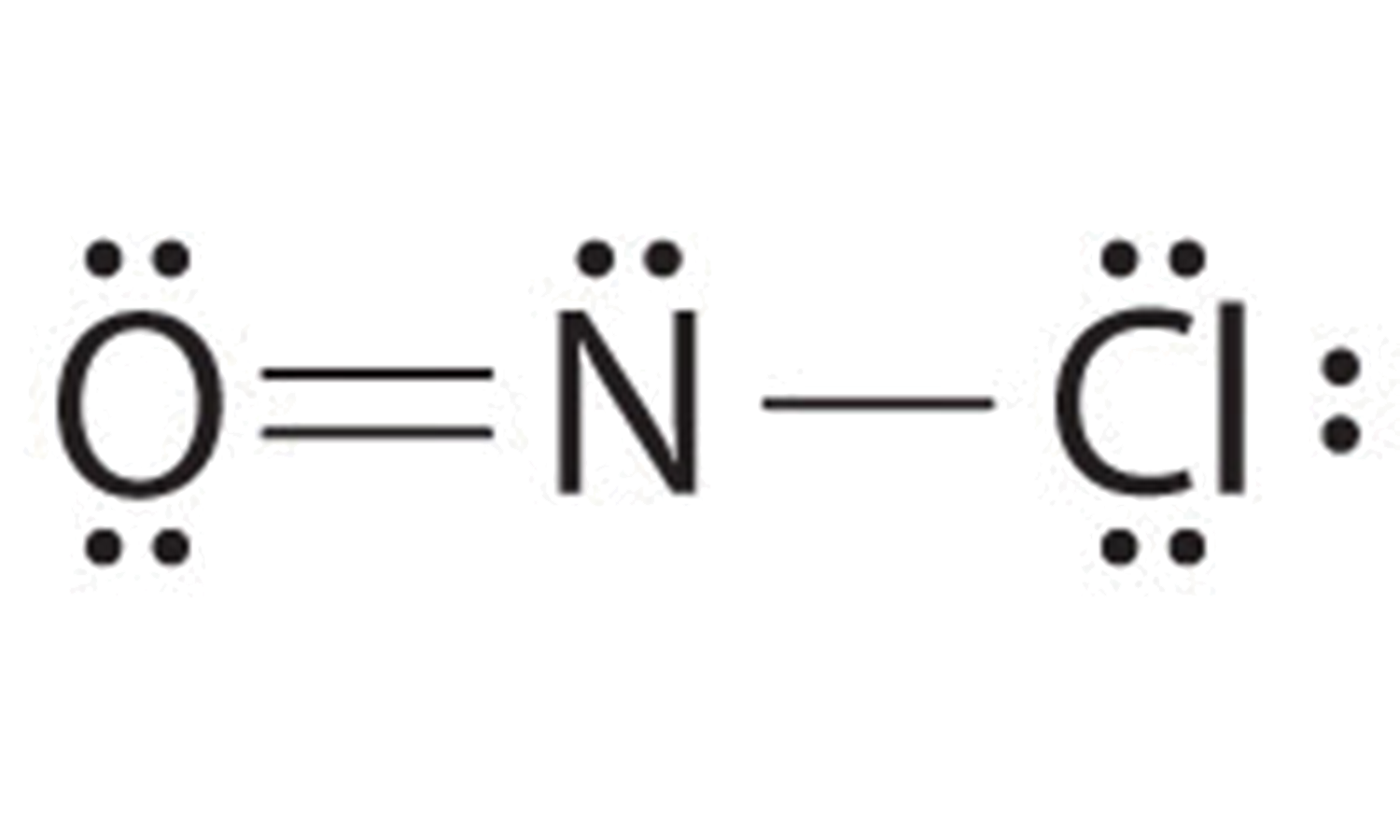
View solution. >. The skeletal structure of C H 3 C O O H as shown above is correct but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid. Medium. View solution. >. Chlorine is represented by the Lewis structure (image). The atomic number of an atom that will give an identical electron-dot arrangement as ...
Lewis Dot Structures. During chemical bonding it is the valence electrons which move amongst different atoms. In order to keep track of the valence electrons for each atom and how they may be shared in bonding we use the Lewis Dot Structure for atoms and molecules. In this approach we represent the valence electrons as dots around the element ...
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The lines are a short-hand version of the two dots representing the covalent bonds.
In a lewis dot diagram, each element will share 8 electrons (there are exceptions like hydrogen and boron) ... electrons. structural formula lone pairs. Diagram that Shows the bonds formed between atoms as well as the lone pairs. sodium chloride (Lewis Dot Diagram. calcium carbonate. nitrogen monoxide. NO. YOU MIGHT ALSO LIKE... 18. Aviation ...
For example, consider sodium chloride. The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells. ... Lewis dot diagrams give us a static picture of what the molecule or ion ...
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
Formation of NaCl The following electron dot diagram shows the formation of NaCl. Formation of MgF 2 The following electron dot diagram shows the formation of MgF 2. Notice that instead of drawing electron dot diagrams for 2 F-, the number “2” has been placed in front of one of the F-.
Show how Lewis dot diagrams also represent ionic bonding. Tell students that dot diagrams can also be used to show ionic bonding. Project the image Ionic bonding of sodium chloride. Ask students: In the second dot diagram, why are there no electrons surrounding sodium? The electron was transferred to chlorine.
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
3.2 NaCl 3.3 AlCl 3, 3.4 PCl 3, 3.5 CaO 3.6 F 2 Question 4 Every day we use table salt to season our food. Table salt is the common name for sodium chloride. 4.1 Give the chemical formula for sodium chloride 4.2 Draw Lewis dot diagrams for the sodium ion (Na+) 4.3 Draw Lewis dot diagrams for the chlorine ion (Cl-)
What is the electron dot structure for NaCl? Sodium atom will loose one electron to gain noble gas configuration and form sodium cation with +1 charge. Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge. In sodium chloride the one electron from sodium metal gets transferred to chlorine atom.


























0 Response to "38 nacl lewis dot diagram"
Post a Comment