37 orbital diagram for vanadium
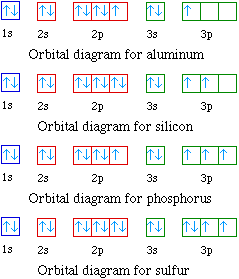
An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up) Cr and Cu, as well as Cu and Ag, are exceptions in the "typical" filling order.Orbital Diagram. 1s Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Sources Found in the minerals patronite (VS4), vanadinite [Pb5 (VO4)3Cl], and carnotite [K2 (UO2)2 (VO4)H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is .
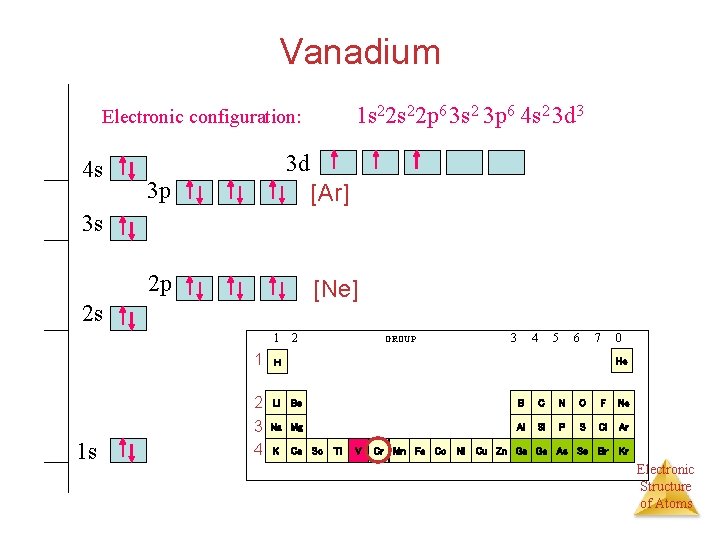
The orbital diagram for hydrogen can be represented in the following way. ... As electrons are added in titanium and vanadium the configuration is [Ar]4s 2 3d 2 and [Ar]4s 2 3d 3. The next element, chromium, would be expected to have a configuration of [Ar]4s 2 3d 4, however this is not the case. It turns out that as a result of the similarity ...
Orbital diagram for vanadium
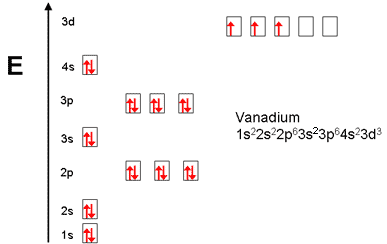
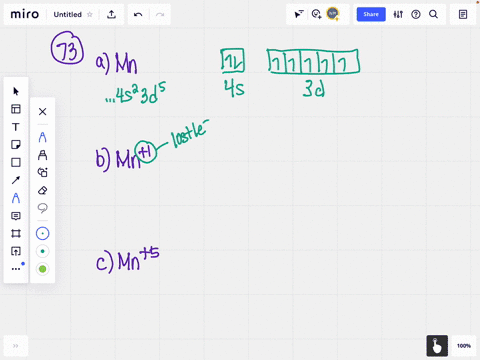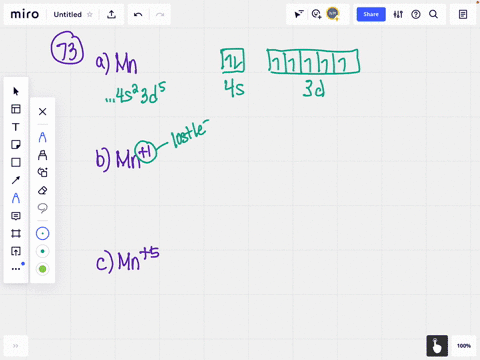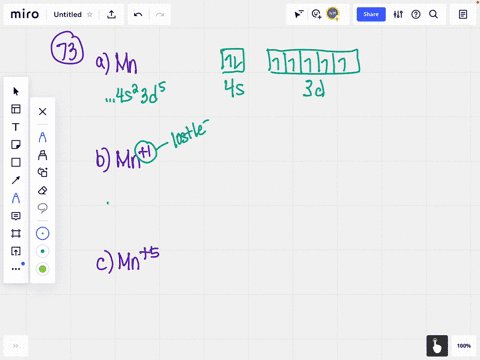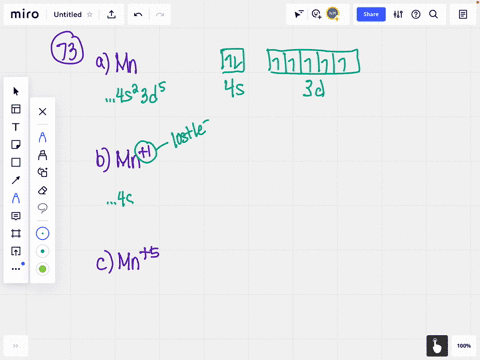
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom. The orbital diagram of vanadium is: Vanadium has the ability to exist as a +5,2,3,4 oxidation state and has a very special electronic configuration. The vanadium consist of added electrons to its ... In the case of Vanadium the abbreviated electron configuration is [Ar] 3d3 4s2. Nevertheless, check the complete configuration and other interesting facts about Vanadium that most people don't know. Found in the minerals patronite (VS4), vanadinite [Pb5 (VO4)3Cl], and carnotite [K2 (UO2)2 (VO4)2.3H2O].
Orbital diagram for vanadium. Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration February 18, 2021 by Sneha Leave a Comment Vanadium Electron Configuration : When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned before in our article. What is the orbital diagram for Vanadium? [Ar] 4s= 1 up 1 down. Next orbital: 3 up arrows. Which set of four quantum numbers corresponds to an electron in a 4p orbital? A.) n=4, l=1, ml=0, ms=1/2 B.) n=4, l=3, ml=3, ms=-1/2 C.) n=4, l=2, ml=0, ms=1/2 D.) n=4, l=4, ml=3, ms=-1/2. A.) n=4, l=1, ml=0, ms=1/2. Which element has the smallest atomic ... Orbital Diagram For Vanadium (V) Vanadium Electron . Answer and Explanation: 1. Vanadium has an atomic number 23. The full electronic configuration of vanadium is. 1s22s22p63s23p63d34s2 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 3 4 s 2 ; Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable ... T1 - Orbital order in vanadium spinels. AU - Di Matteo, S. AU - Jackeli, G. AU - Perkins, N. B. PY - 2005/7/1. Y1 - 2005/7/1. N2 - Motivated by recent theoretical and experimental controversy, we present a theoretical study to clarify the orbital symmetry of the ground state of vanadium spinel oxides AV2O4 (A=Zn, Mg, Cd).
Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Vanadium has 23 electrons. To simplify the ground-state electron configuration of ... Orbital Diagram. 1s Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Sources Found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl], and carnotite [K2(UO2)2(VO4)H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is .Orbital Filling Electron Configurations Where do these electrons go? - ppt downloadwiringall.com: Vanadium: Orbital and Bonding ... Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34 ...
Drawing electron configuration diagrams | Chemistry for All | The Fuse Electron Configuration For Vanadium - V, V2+, V3+, and V5+ Ions Quantum Numbers Explained - Electron Configuration, Atomic Orbital Diagrams - S P D F & n l ml ms . Pourbaix diagrams are useful in predicting the stabilities of various species. Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration February 18, 2021 by Sneha Leave a Comment Vanadium Electron Configuration: When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned before in our article. Orbital Diagram For Vanadium V Vanadium Electron Configuration Periodic Table . And thus V X 3 is paramagnetic because it has two unpaired 3 d -electrons. Vanadium iv electron configuration. First Ionization Energy of Vanadium is 67463 eV. Possible oxidation states are 2345. The chemistry of vanadium is noteworthy for the accessibility of the ... Vanadium-steel alloys are very tough and are used for armour plate, axles, tools, piston rods and crankshafts. Less than 1% of vanadium, and as little chromium, makes steel shock resistant and vibration resistant. Vanadium alloys are used in nuclear reactors because of vanadium's low neutron-absorbing properties.
The valency depends on the bond formation. Vanadium 4 and 5 valency is used most of the time. How many valence electrons does vanadium ion(V 2+, V 3+) have? The electron configuration of vanadium shows that the last shell of vanadium has two(4s 2) electrons and the d-orbital has a total of three electrons. There are two types of vanadium ions.
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. wiringall.com!
Orbital Diagram. 1s ... Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Sources Found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl], and carnotite [K2(UO2)2(VO4)2.3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere.
Electron configuration for Vanadium (V) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1. Electron configuration for Yttrium (Y) Carbon (C) What element is represented by this orbital diagram? ... What element is represented by this orbital diagram? THIS SET IS OFTEN IN FOLDERS WITH... 6.2 Classifying Elements. 17 terms. mnethercott. SVHS Chem 8.1 ...
This video shows on how to write the orbital diagram of V2+ and V3+ ions.
Defect molecular orbital diagram for vanadium (h) and vanadium (k) in 4H SiC under C3v symmetry regarding only the crystal-field splitting. Characters of all Kohn-Sham levels are indicated.
Vanadium (V) has an atomic mass of Find out about its Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. ↿⇂ Planetary Bohr Model of Vanadium (V). Description: Soft. Vanadium is highly resistant to corrosion, and thus is commonly added to stainless steel alloys. Vanadium compounds are used in advanced. 23_vanadium_(V)diagramweb.net ( × pixels, file size: 37 KB, MIME type: image/png). Open in ...
Electron orbital diagram of vanadium [duplicate] Ask Question Asked 5 years, 9 months ago. Active 4 years ago. Viewed 30k times 2 $\begingroup$ This question already has answers here: Why do elements in columns 6 and 11 assume 'abnormal' electron configurations? (4 answers) Closed 4 years ago. I am learning about electron configurations and I came across the "exceptions" of $\ce{Cr}$ and $\ce ...
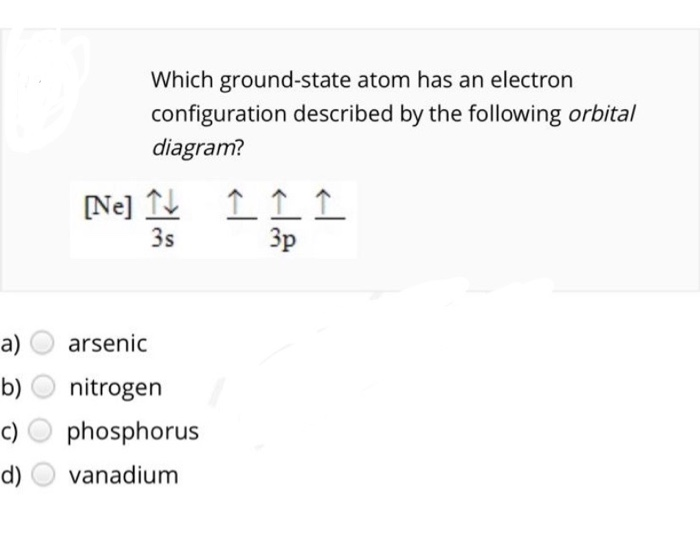
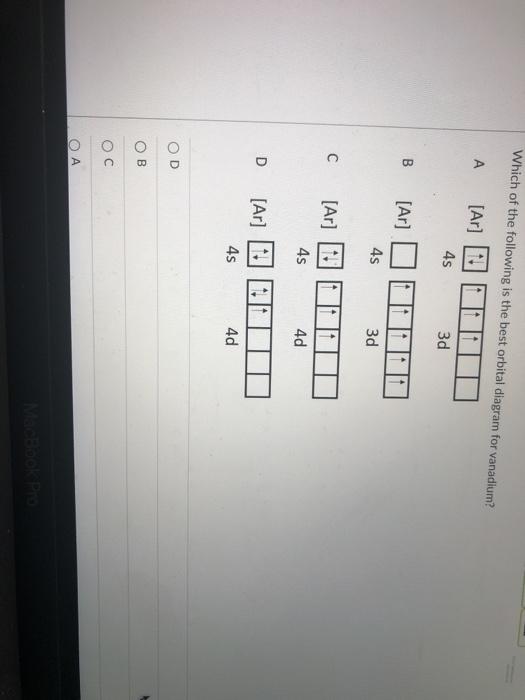
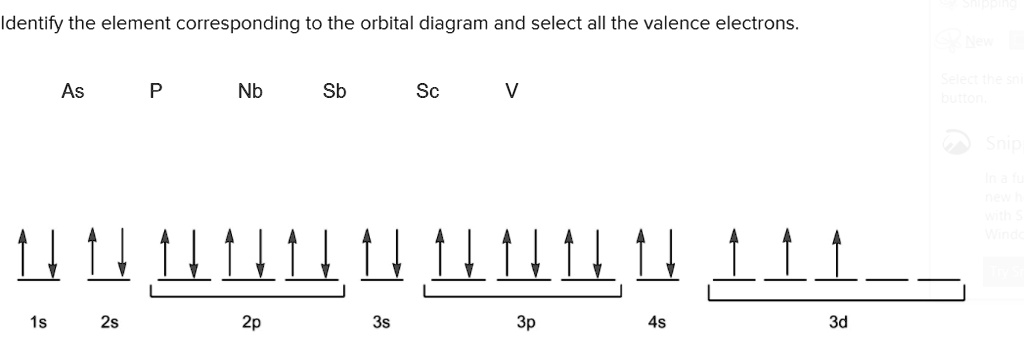
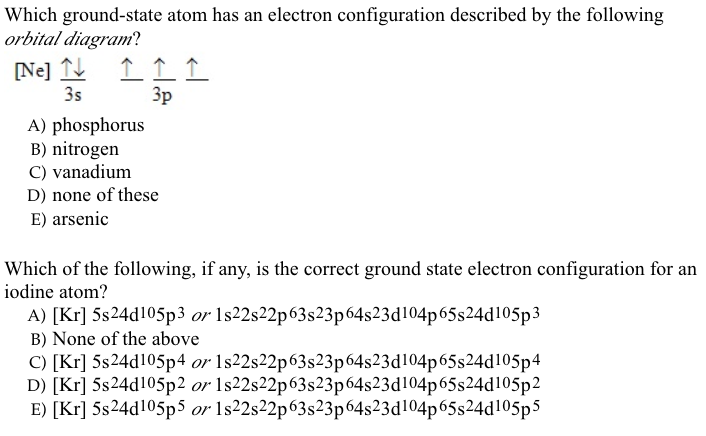
We're being asked to choose the correct orbital diagram for vanadium. For that, we first need to determine the electron configuration of Vanadium. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Vanadium is 23 and since it's a neutral element, this means V has 23 electrons. 86% (432 ...
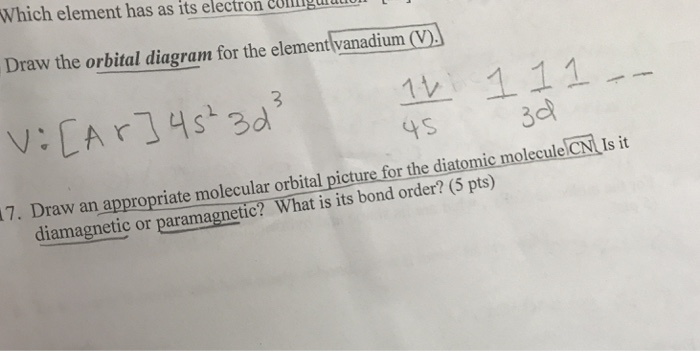
How to choose the correct orbital diagram for vanadium? Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4. Electrons Per Shell, 2 8 11 2. Electron Configuration, [ Ar] 3d3 4s2. If playback doesn’t begin shortly, try restarting your device.
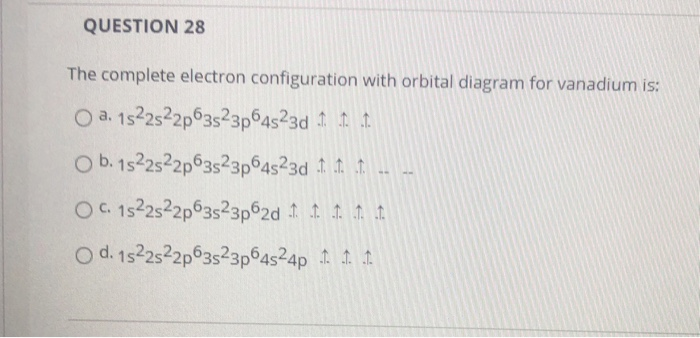
Vanadium is a chemical element with atomic number 23 which means there are 23 protons and 23 electrons in the atomic structure.The chemical symbol for Vanadium is V. Electron Configuration and Oxidation States of Vanadium. Electron configuration of Vanadium is [Ar] 3d3 4s2. Possible oxidation states are +2,3,4,5. Electron Configuration
In the case of Vanadium the abbreviated electron configuration is [Ar] 3d3 4s2. Nevertheless, check the complete configuration and other interesting facts about Vanadium that most people don't know. Found in the minerals patronite (VS4), vanadinite [Pb5 (VO4)3Cl], and carnotite [K2 (UO2)2 (VO4)2.3H2O].
The orbital diagram of vanadium is: Vanadium has the ability to exist as a +5,2,3,4 oxidation state and has a very special electronic configuration. The vanadium consist of added electrons to its ...
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.

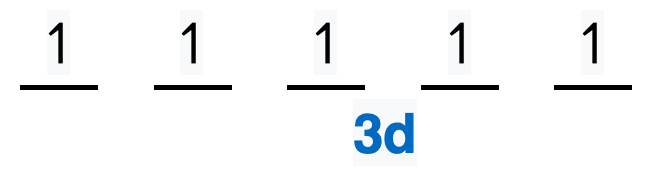

















0 Response to "37 orbital diagram for vanadium"
Post a Comment