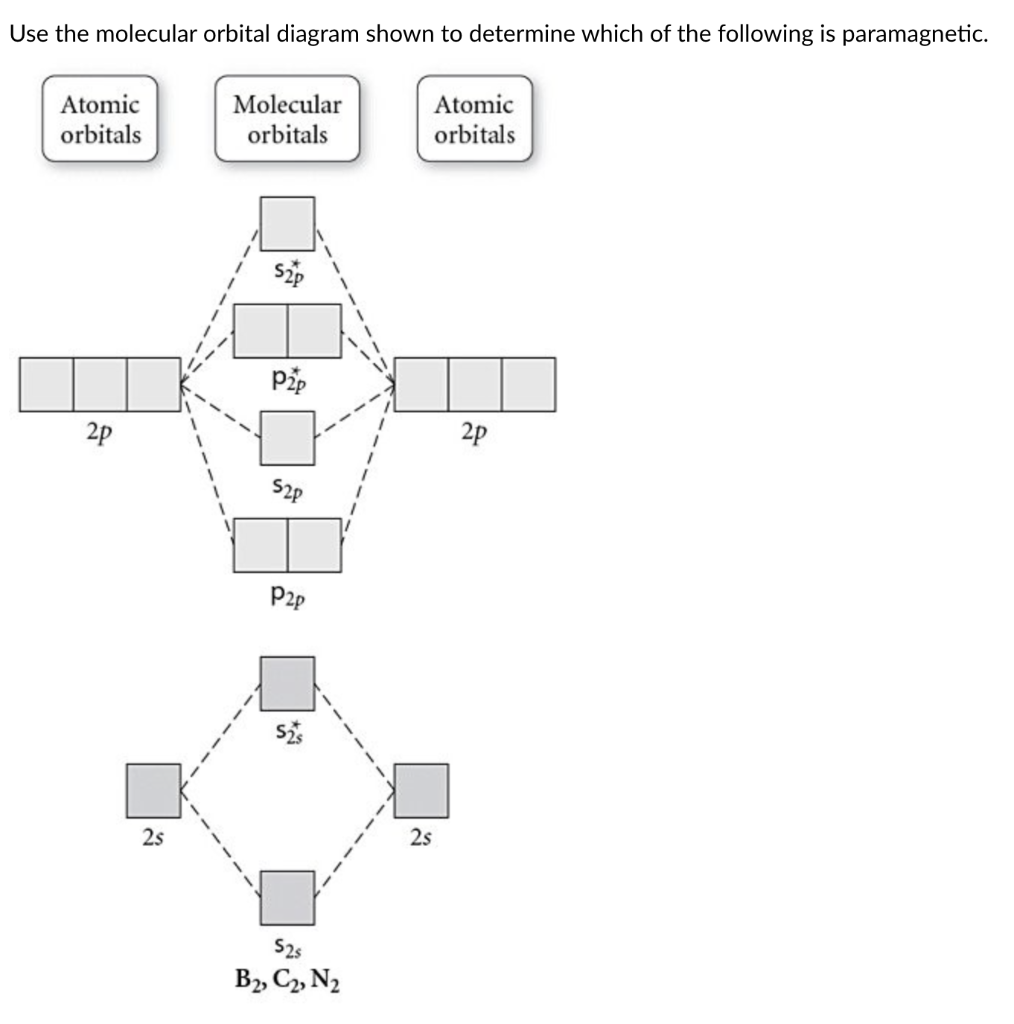
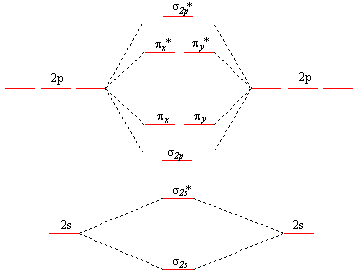
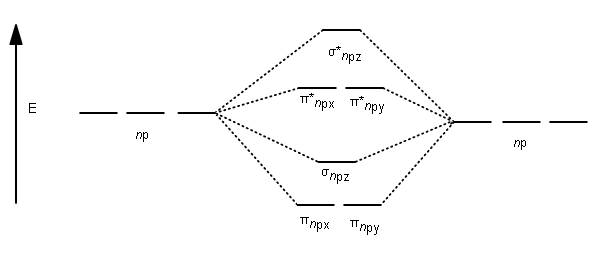
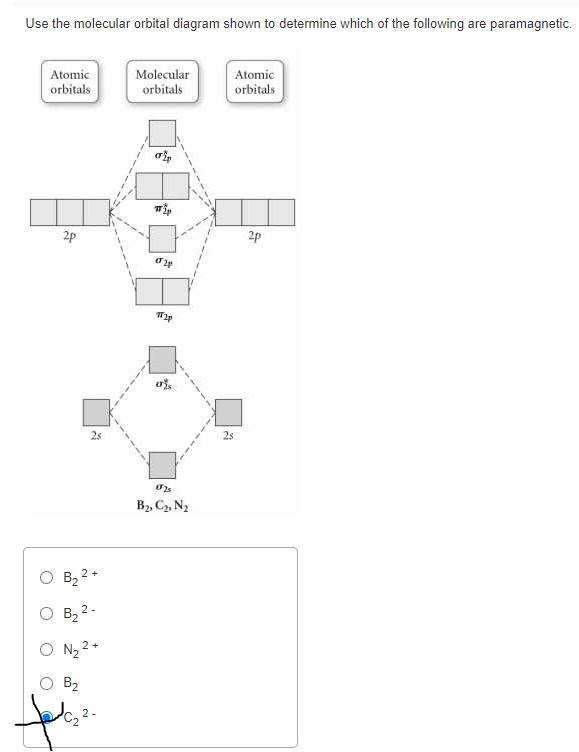
38 draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
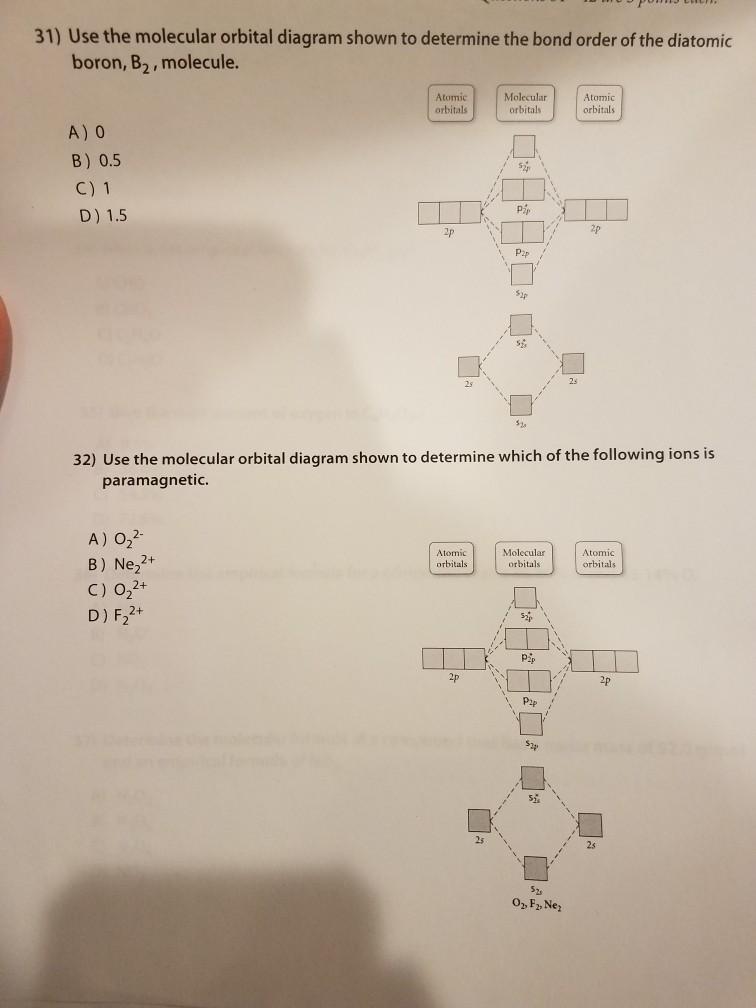
Quiz 1 Flashcards | Quizlet 2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2. study.com › learn › electron-configuration-questionsElectron Configuration Questions and Answers | Study.com Draw and label the orbital drawing for the highest energy d orbital electrons in dubnium. View Answer Show the full ground-state electron configuration of arsenic by building its orbital diagram.
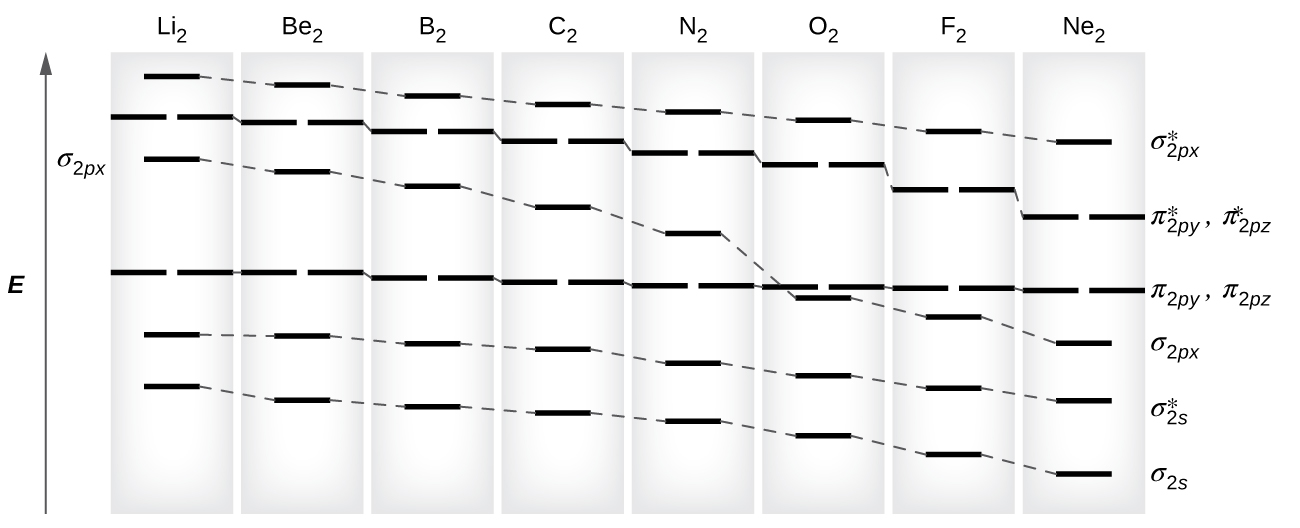
support.ebsco.com › LEX › AP-Chemistry_Study-GuideAP Chemistry Study Guide - EBSCO Connect orbital; thus, a single orbital can only have one spin up and one spin down electron. When subshells are not filled, unpaired electrons are present and can interact with magnetic fields, making the atom paramagnetic. When all subshells are filled, the element does not interact with magnetic fields and is called diamagnetic.
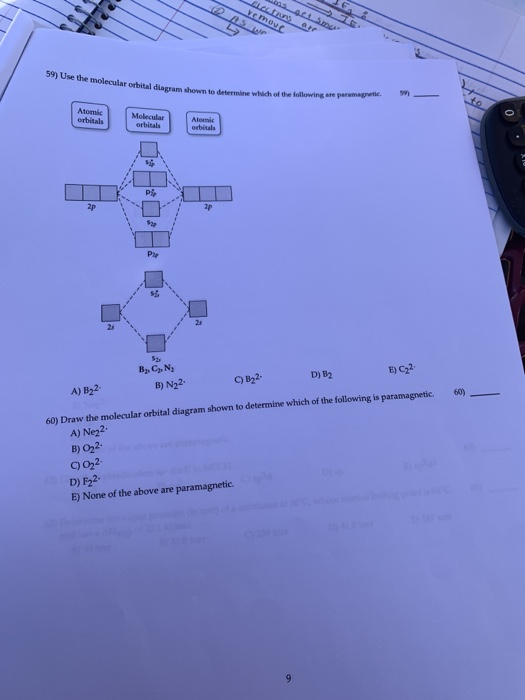
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
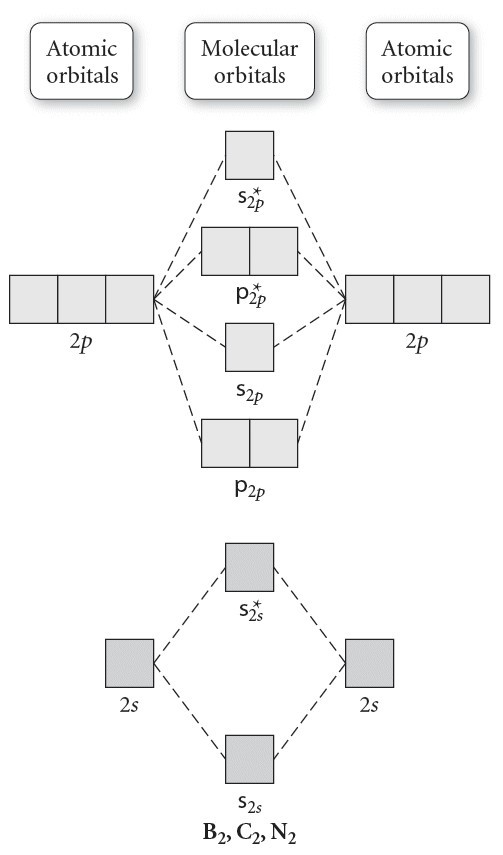
Use the molecular orbital diagram shown to determine which ... 57) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) B 2 2 ⁺ B) B 2 2 ⁻ C) N 2 2 ⁺ D) C 2 2 ⁻ E) B 2 Answer: E C ) B 2 The term paramagnetism depends upon the number of unpaired electrons. More the number of unpaired electrons, more will be the paramagnetism. Study Chapter 4 Pre-Exam Flashcards - Quizlet Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B₂ Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 Answers The one that is most stable is F2 or E Explanation: The question is incomplete.
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.. Solved Draw the molecular orbital diagram shown to ... See the answer Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Answer options: B2 B22+ N22+ C22- B22- Expert Answer 98% (45 ratings) Previous question Next question Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages E) None of the above are paramagnetic At six or twelve valence electrons, it is paramagnetic 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2- C) N2^2+ D) C2^2- E) B2 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ › 36835743 › Problems_and_Solutions(PDF) Problems and Solutions on Atomic ... - Academia.edu Academia.edu is a platform for academics to share research papers. Answered: What is the bond order in LiF? A. 0 B.… | bartleby The following molecular orbital diagram belongs to a molecule of the type XH2 (where X is an unidentified 2nd row element). Do you think the molecule is bent or linear? Explain your reasoning. Make approximate sketches of the bonding and non-bonding molecular orbitals.
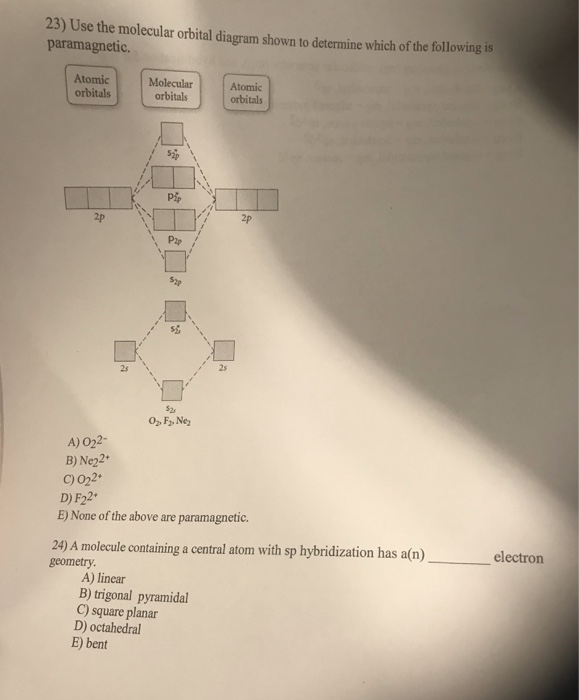
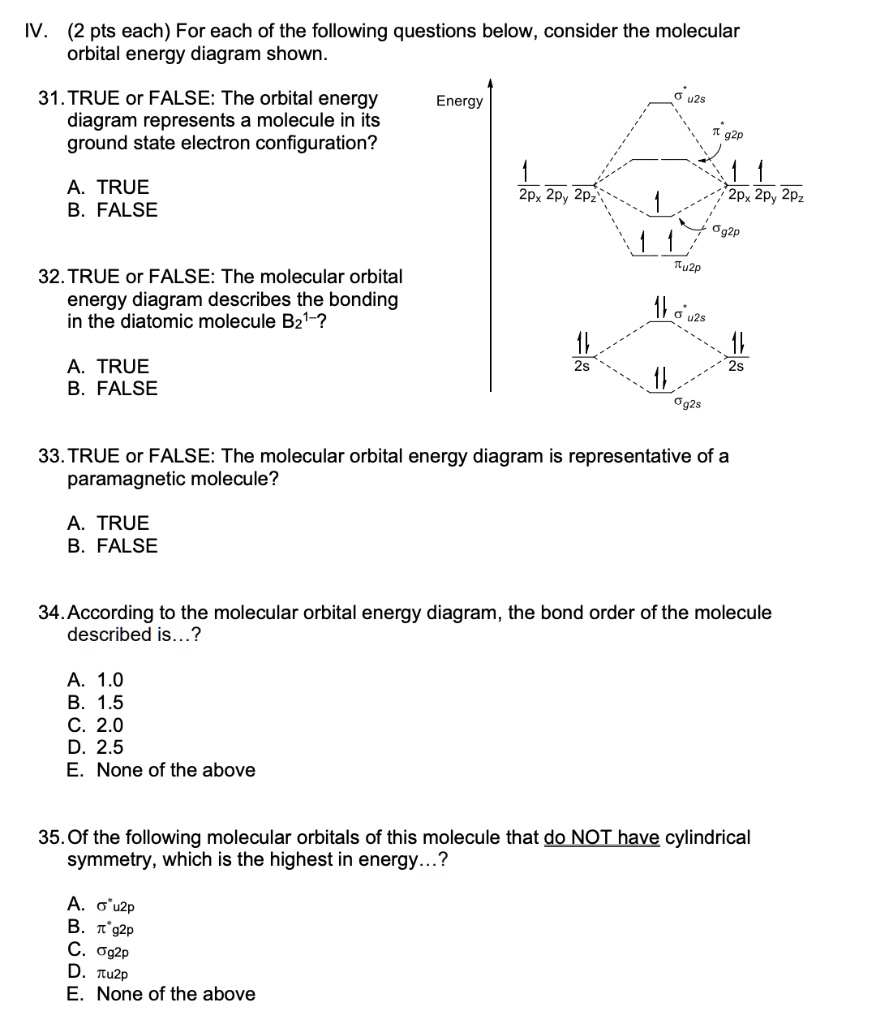
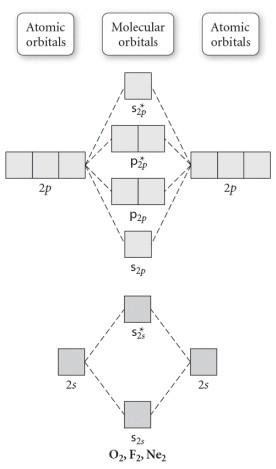
quizlet.com › 463449990 › chem-c125-final-examChem-C125 Final Exam Review Flashcards | Quizlet Assume that the energy needed for an electron in 2p orbital in an O atom to jump to 3s orbital is 3.88*10-19 J, what is its wavelength of the line atomic spectra in nanometer (nm)? 512 Given: In Atomic Spectra lab, a student obtained his best-fit line equation to be y = 0.29 x + 46.8 when he plotted his Vernier reading on the y-axis and ... Doc 117 b p s xi chemistry iit jee advanced study package ... 5.9.2016 · Read Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by S.Dharmaraj on Issuu and browse thousands of other publications on our p... 40 o2+ molecular orbital diagram - Wiring Diagrams Manual Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Use molecular orbital diagrams to predict which of the ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic Given that O2 is paramagnetic and has a bond order of 2, and its highest occupied molecular orbital is antibonding, what would be the expected bond orders for O22- and O22+?
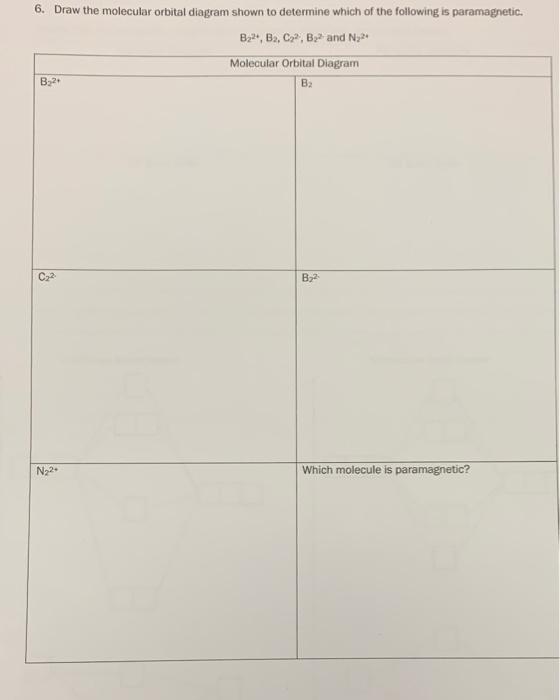
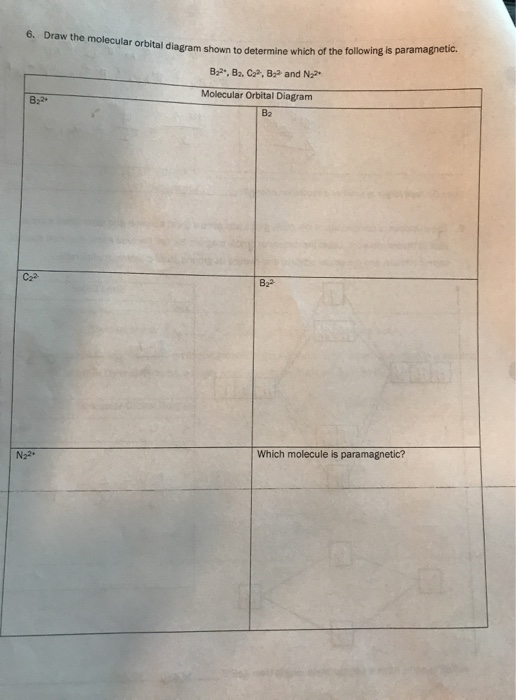
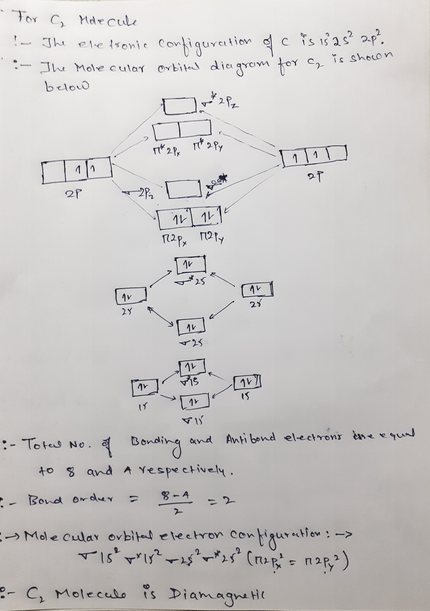
› 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham Draw the molecular orbital diagram shown to determine ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22- For each of the following examples, (a) Draw a molecular orbital diagram. Include both the atomic... For each of the following examples, (a) Draw a molecular orbital diagram. Solved 6. Draw the molecular orbital diagram shown to ... 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B2, B, C, B and N, Molecular Orbital Diagram B B24 C2 B2 N22 Which molecule is paramagnetic? Question: 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. quizlet.com › 185712467 › chemistry-1211-flash-cardschemistry 1211 Flashcards - Quizlet Determine the number of valence electrons in SO₃ and then draw the corresponding Lewis structure (by following the octet rule on all atoms). 24 The answer to the calculation below with the correct number of significant figures is
EOF
Answered: Draw the molecular orbital diagram… | bartleby Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+ Question Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B 22+, B 2, C 22-, B 22-, and N 22+ Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution
Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 Answers The one that is most stable is F2 or E Explanation: The question is incomplete.
Study Chapter 4 Pre-Exam Flashcards - Quizlet Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B₂ Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Use the molecular orbital diagram shown to determine which ... 57) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) B 2 2 ⁺ B) B 2 2 ⁻ C) N 2 2 ⁺ D) C 2 2 ⁻ E) B 2 Answer: E C ) B 2 The term paramagnetism depends upon the number of unpaired electrons. More the number of unpaired electrons, more will be the paramagnetism.
















0 Response to "38 draw the molecular orbital diagram shown to determine which of the following is paramagnetic."
Post a Comment