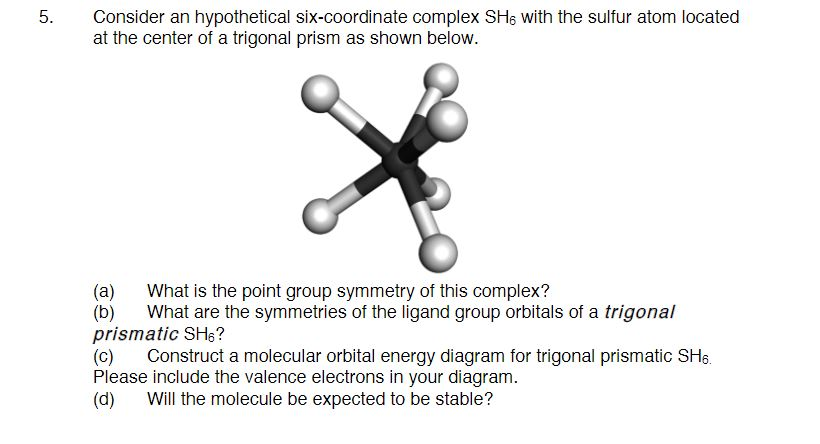
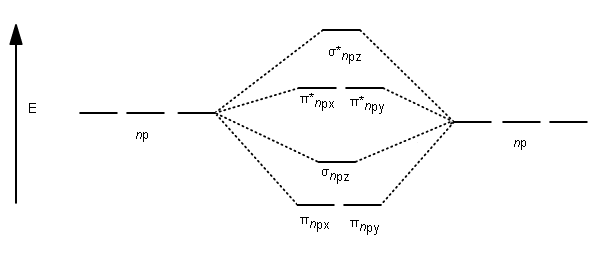
38 sh6 molecular orbital diagram
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. 2.7 Molecular Orbital Theory - Inorganic Chemistry for Chemical... Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes The electrons occupying the sH-H orbital represent the bonding pair of electrons from the Lewis structure of H2 and is aptly named a bonding molecular In the middle of the diagram, the molecular orbitals of the molecule of interest are written. Dashed lines connect the parent atomic orbitals with...
Sh6 molecular orbital diagram
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the One of the molecular orbitals in this molecule is constructed by adding the mathematical functions The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both... The Orbitron: a gallery of atomic orbitals on the WWW Molecular orbitals. coming soon... The Orbitron. Images representing atomic orbitals and a few molecular orbitals Animated plots of wave functions The Pi Molecular Orbitals of Butadiene And How To Draw Them The Lowest-Energy Molecular Orbital (π1) Of The Butadiene Pi System Has Zero Nodes The Full Molecular Orbital Diagram For The Butadienyl System (n=4) A molecular orbital diagram without electrons is like an apartment building without people.
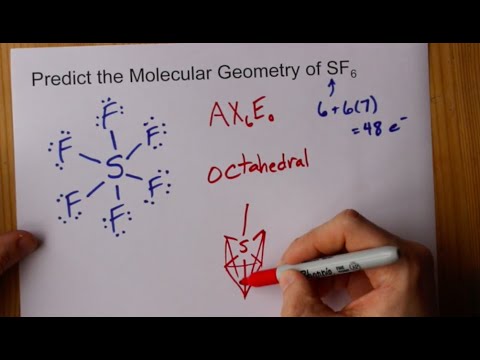
Sh6 molecular orbital diagram. PDF Organometallic Chemistry 6The molecular orbital diagram illustrated in Figure 2-4 considers only interactions between atomic orbitals of identical energy: 2s with 2s and 2p with 2p. A Suggested Procedure for Writing Molecular Orbital Diagrams. The molecular orbital concept is fundamental to organometallic chemistry. Introduction to Molecular Orbital Theory The upper molecular orbital has a node in the electronic wave function and the electron density is low between the two positively charged nuclei. This section illustrates pictorially molecular orbitals for several organic and inorganic molecules. If possible - the energy level diagram is included and... Molecular orbital diagram, octahedral transition metal complex 6. Molecular orbital diagram for an octahedral transition metal complex MLe illustrating different types of electronic transitions based on localized orbital configurations (MC, metal-centered LC, ligand-centered MLCT, metal-to-ligand charge transfer LMCT, ligand-to-metal charge transfer). 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine.
PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (Heteronuclear Diatomics) The molecular orbital diagram for the heteronuclear diatomic compound nitrogen monoxide, NO, is 9.6 Molecular Orbital Theory bonding molecular orbital antibonding molecular orbital molecular orbital diagram bond order. Chapter 9. PDF PowerPoint Presentation u Stable electronic configurations: MO Energy Level Diagrams Reviewed u Electron count preference u Electron count and Oxidation States u Ligands. • Carbon Monoxide • Phosphines • Cyclopentadienide and arenes • Hydrides and dihydrogen. Molecular Orbital Diagrams (π-bonding) Part 4 Molecular Orbital Theory. Part 5 Coordination Chemistry. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
What is the molecular orbital diagram for NO? - Quora Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as. Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Fill the molecular orbitals in the energy-level diagram beginning with the orbital with the lowest energy. Be sure to obey the Pauli principle and Hund's rule while doing so. PDF Microsoft PowerPoint - Molecular Orbital Theory_ the best of... • Molecule orbital theory (Robert Mullikan). • Assumes electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital... Molecular Orbital Diagram For Co - Wiring Site Resource Symmetry of orbital interactions. The y axis of a mo diagram represents the total energy not potential nor gibbs energy of the orbitals.
Chapter 6 - Molecular Structure Chapter 6 - Molecular Structure. Introduction. A method for constructing Lewis structures of simple molecules and ions was presented in Chapter 5. In this chapter A molecule is a three-dimensional structure, and many of its properties, both chemical and physical, are dictated by that structure.
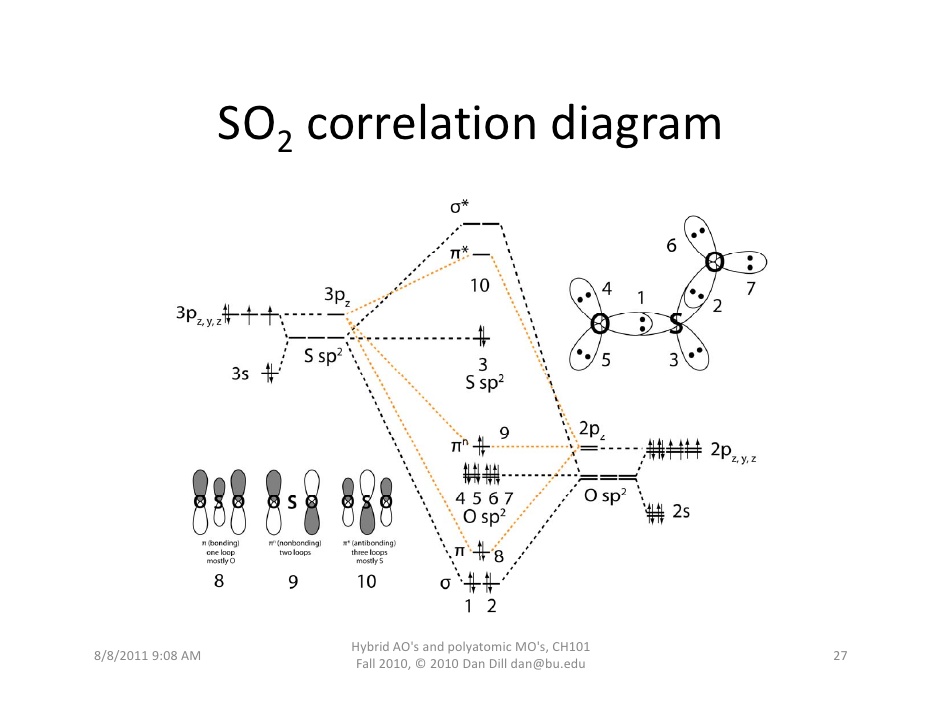
Molecular Orbital Theory Diatomic molecules: Heteronuclear... 10 Molecular Orbital Theory Polyatomic molecules If you are trying to estimate the appropriate geometry for a triatomic molecule using a Walsh diagram, all that is necessary is to correctly determine the number of electrons that will populate the orbitals in the diagram. Then you can estimate which...
Molecular Orbital Diagrams -- Chemistry X - YouTube For chem videos, quizzes and more download Chemistry X for free on the App Store! Correlation Diagrams - by considering the positions and energies of...
Atomic and Molecular Orbital Diagram for Oxygen/O2 Describe molecular bonding using molecular orbital theory, compare/contrast with VESPR and Lewis theory. Understand how to draw & fill molecular orbital diagrams (Pauli Remembering how to draw the MO diagram for B,C,N Vs O,F,Ne - using dry erase board, generic diagram handout, or other.
Molecular-Orbital Theory - PDF Free Download Molecular-Orbital Theory 1 Introduction Orbitals in molecules are not necessarily localized on atoms or between atoms as suggested in the valence bond 6 Molecular Orbital Diagrams for Second Row Diatomic Molecules B 2 through C 2 6 Notes: For B through N, 2s and 2p are close enough in energy...
How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy Dot diagrams are very different to orbital diagrams, but they're still very easy to understand. They consist of the symbol for the element in the...
Molecular orbital diagram — Wikipedia Republished // WIKI 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that...
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it.
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or Heteronuclear Diatomic Species The following is a molecular orbital energy level diagram for a heteronuclear diatomic molecule, XY, in...
PDF Procedure for Constructing Molecular Orbital Diagrams Based on... Molecular orbitals extend over the entire molecule although there can be more electron density in particular regions. We could use the symmetry-based method to construct molecular orbital diagrams for larger molecules as well, but this can get complicated for larger structures.
Figure 2: The Molecular Orbital Diagram of O2 Molecule Key Terms: Antibonding Molecular Orbital, Atomic Orbital, Bonding Molecular Orbital, Hybridization, Hybrid Orbital, Molecular Orbital. sp2 Hybrid Orbital. These orbitals are formed when one s orbital and 2 p orbitals are hybridized. The resulting hybrid orbitals have about 33% of s characters and...
Molecular Orbital Diagram For Cn - Free Catalogs A to Z The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. Category: University Templates Show details. Lecture 6 4 coordinate complexes, summary, typical exam. 1 hours ago Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2...
The Pi Molecular Orbitals of Butadiene And How To Draw Them The Lowest-Energy Molecular Orbital (π1) Of The Butadiene Pi System Has Zero Nodes The Full Molecular Orbital Diagram For The Butadienyl System (n=4) A molecular orbital diagram without electrons is like an apartment building without people.
The Orbitron: a gallery of atomic orbitals on the WWW Molecular orbitals. coming soon... The Orbitron. Images representing atomic orbitals and a few molecular orbitals Animated plots of wave functions
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the One of the molecular orbitals in this molecule is constructed by adding the mathematical functions The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...


![7. [24 pts] Consider the hypothetical hexagonal | Chegg.com](https://media.cheggcdn.com/study/09b/09bbfd74-988b-4a3f-a14f-95ae74ae45a5/image.png)







![How is the bonding in the [Au6C(PPh3)6]2+ cluster explained ...](https://i.stack.imgur.com/Y1psc.png)








0 Response to "38 sh6 molecular orbital diagram"
Post a Comment