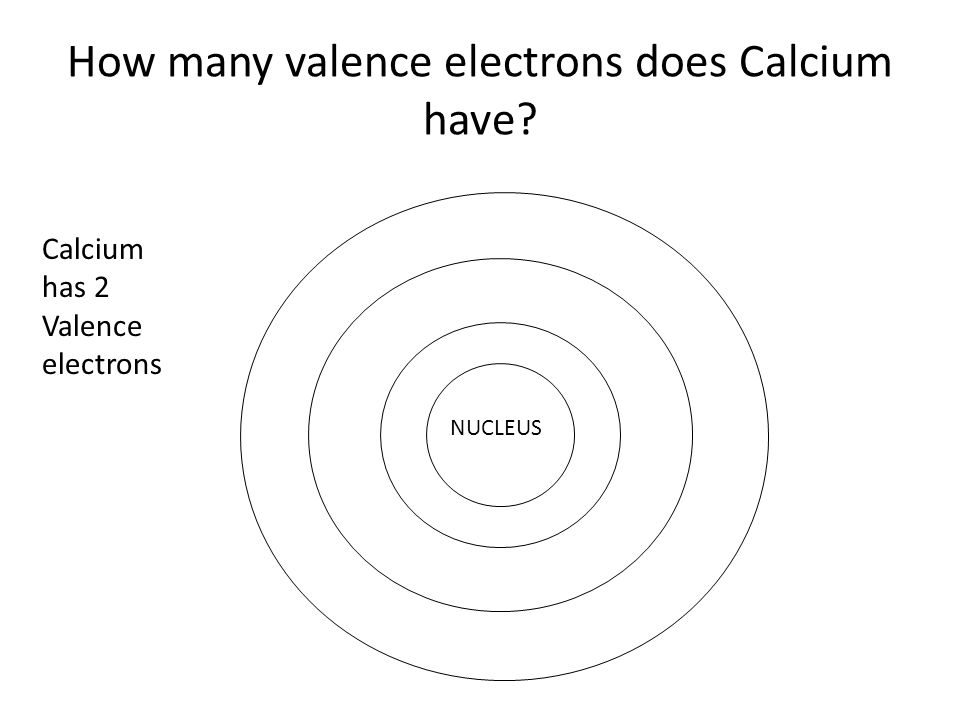
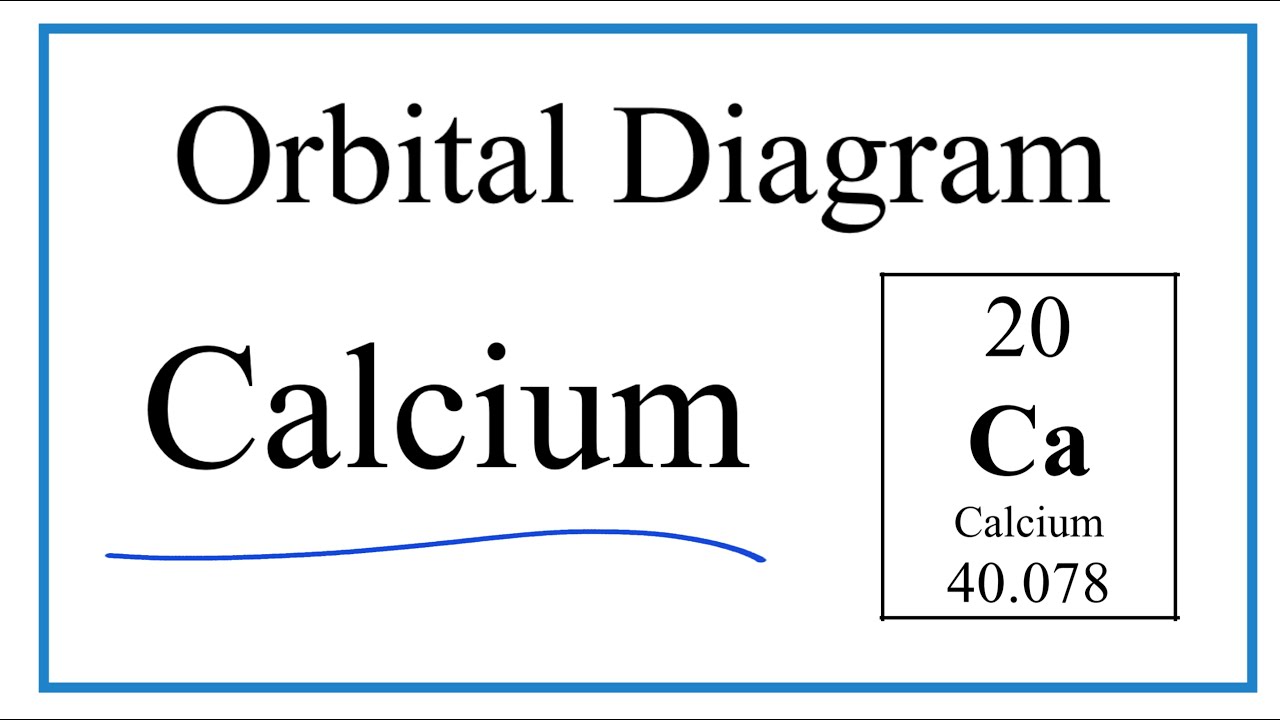
37 orbital filling diagram for calcium
Orbital Filling Diagram For Sulfur Stack the subshells in order of energy, with the lowest-energy subshell. Well, we use the aufbau principle, and for sulfur, Z=Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p⁶3s²3p⁴. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. Orbital Diagram For Vanadium (V) | Vanadium Electron ... Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free of cost and no charges are ...
Orbital Filling Diagram For Calcium Orbital diagrams are pictorial representations of the arrangement of Rather than filling the orbitals one-by-one, it is easier to note that calcium is in the s- block. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Here I use the electron.
Orbital filling diagram for calcium
Orbital filling diagrams - The Cavalcade o' Chemistry The rules for orbital filling diagrams. If you want to learn how to draw orbital filling diagrams, you need to follow these handy rules. They probably won't make sense right now, but I'll explain them when the time is right. For now, trust me that these rules are handy ones: Electron configurations list the orbitals from lower to higher energy. For example, when I show you the electron configuration for oxygen (1s²2s²2p⁴), this means that the 1s orbital is lowest in energy, followed ... PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e-•Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom chemed.chem.purdue.edu › genchem › topicreviewQuantum Numbers and Electron Configurations Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. There is one orbital in an s subshell (l = 0), three orbitals in a p subshell (l = 1), and five orbitals in a d subshell ...
Orbital filling diagram for calcium. slestories.nl › wvoovRunuo scripts - slestories.nl Mar 05, 2022 · In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. 60 seconds. Bohr model worksheet key. Showing top 8 worksheets in the category - Ground State Vs Excited State. absorbs energy as it moves to a higher energy state. plPogil activities for ap chemistry fractional precipitation Ap classroom ... How to Draw Orbital Diagrams for any atom | Orbital Notation The orbital diagram is drawn by using three rules - Aufbau's rule, Hund's rule, and Pauli's exclusion rule. For drawing the orbital diagram or orbital notation, first, find the number of electrons in an atom then write its electron configuration to determine which orbital should be filled. And then fill the electrons in empty orbital ... SOLVED:Apply the Pauli exclusion principle, the aufbau ... That's the electron configuration of calcium, which we know by filling in this diagram and now krypton. Now, krypton is definitely more difficult. And the reason for this is because krypton, um is it's beyond. It's further down the period from calcium, meaning that it's going to have de, um, electron orbital's. PDF 2.Draw Orbital Diagram for following Elements 2.Draw Orbital Diagram for following Elements Elements Orbital filling of Elements Aluminium Calcium Silicon Potassium Chlorine 3.Mark the Electronic Configuration of the elements Magnesium Sodium Fluorine Boron Lithium 9 11 12 5 3
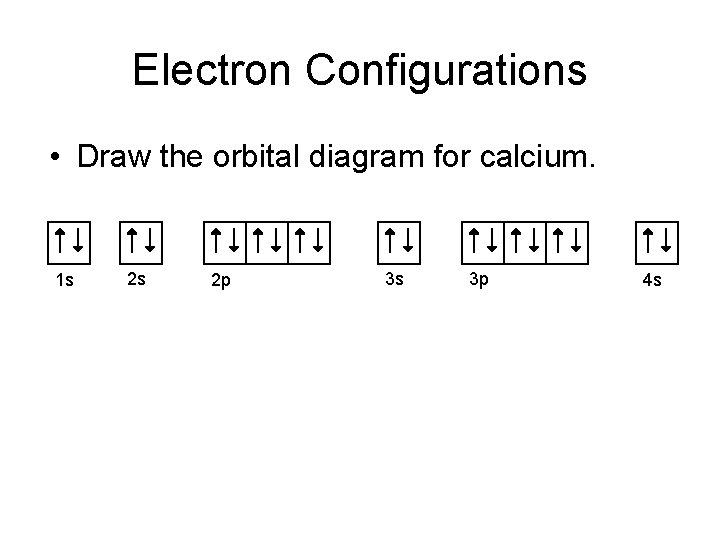
PDF Hund©s Rule & Orbital Filling Diagram Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d How to Do Orbital Diagrams - Sciencing The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals. Hund's Rule and Orbital Filling Diagrams | Chemistry for ... An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. What is the orbital diagram for calcium? | Study.com What is the orbital diagram for calcium? Orbital Diagrams: Orbital diagrams are pictorial representations of the arrangement of electrons within the orbitals in an atom or molecule. The orbitals...
patapum.to.itFlour Mill Rye [4MH368] Search: Rye Flour Mill. What is Rye Flour Mill. Every flour has its own unique properties. Sourdough Rye using your flour and some crushed organic caraway seeds has lifted my Sourdough Rye to a new level!! › articles › s41524/021/00654-xLow-dimensional non-metal catalysts: principles for ... Nov 16, 2021 · The correlation between CO 2 adsorption energy and the p orbital center of Si atoms is also observed for these endohedrally doped Si clusters, i.e., the smaller-size Si cluster has a higher p ... Electron configuration for Palladium (element 46). Orbital ... The order of filling the orbitals with electrons in the Pd atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 8 But in reality, two electrons move from the 5s orbital to the 4d orbital: Electronic configuration of the Palladium atom in ascending order of orbital energies: chemguide.co.uk › atoms › propertiesthe order of filling 3d and 4s orbitals - chemguide The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.
Palladium(Pd) electron configuration and orbital diagram The 4s orbital is now full. Therefore, the next five electrons will enter the 3d orbital in the clockwise direction and the next five electrons will enter the 3d orbital in the anti-clockwise direction. The 3d orbital is now full. so, the next six electrons will enter the 4p orbital just like the 3p orbital. The 4p orbital is now full.
What is the electron configuration, orbital diagram, and ... In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next ...
Sodium(Na) electron configuration and orbital diagram The electron holding capacity of K orbit is 2n 2 = 2 × 1 2 = 2 electrons. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n 2 = 2 × 2 2 = 8 electrons. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n 2 = 2 × 3 2 = 18 electrons. n=4 for N orbit.
Atomic Orbital Diagram of Calcium (2+) Ion - YouTube Atomic Orbital Diagram of Calcium (2+) Ion. Watch later. Share. Copy link. Info. Shopping. Tap to unmute. If playback doesn't begin shortly, try restarting your device. Up next.
PDF Electron Config & Orbital Filling Answer Key (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Ebdrm Cmfiguratbn api (NO 3p LISL a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g.
Orbital Diagram For Carbon (C) | Carbon Electron Configuration Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any element we ...
Solved Table 3. Electronic configuration of metal ions ... Electronic configuration of metal ions Compound Chemical Metal lon Formula Present Abbreviated Electron Configuration Orbital-filling Diagram for Abbreviated Electron Configuration # of unpaired electrons Magnetism (para or dia) Calcium Sulfate CaSO4 Ca2 [Ne]3s23p6 11111111 0 dia 3s 3p Cobalt (11) Sulfate CoSO4 Co2+ Copper (11) Sulfate CuSO4 Cu2+
Electron configuration for Cerium (element 58). Orbital ... The order of filling the orbitals with electrons in the Ce atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f2. But in reality, one electron moves from the 4f orbital to the 5d orbital:
Solved Fill in the orbital energy diagram for the calcium ... Fill in the orbital energy diagram for the calcium ion. 3p - E3s - The lowest E levels are already filled in for you. Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next.
Essay Gram – We are your custom essay writing service that ... It is very easy. Click on the order now tab. You will be directed to another page. Here there is a form to fill. Filling the forms involves giving instructions to your assignment. The information needed include: topic, subject area, number of pages, spacing, urgency, academic level, number of sources, style, and preferred language style.
Calcium Orbital Filling Diagram - schematron.org Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital.
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Calcium Orbital Filling Diagram Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals.
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11
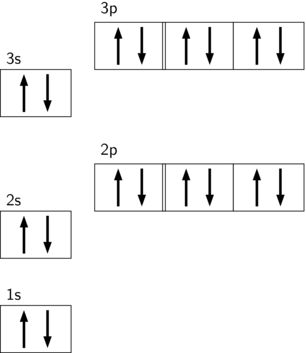
Electron Configuration for Calcium (Ca) In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Calcium Orbital diagram, Electron configuration, and ... What is the orbital diagram for Calcium (Ca)? The orbital diagram for Calcium is drawn with 6 orbitals. The orbitals are 1s, 2s, 2p, 3s, 3p, and 4s. The Calcium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, the next six electrons in 3p orbital, and the remaining two electrons will go in 4s orbital.
chemed.chem.purdue.edu › genchem › topicreviewQuantum Numbers and Electron Configurations Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. There is one orbital in an s subshell (l = 0), three orbitals in a p subshell (l = 1), and five orbitals in a d subshell ...
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e-•Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Orbital filling diagrams - The Cavalcade o' Chemistry The rules for orbital filling diagrams. If you want to learn how to draw orbital filling diagrams, you need to follow these handy rules. They probably won't make sense right now, but I'll explain them when the time is right. For now, trust me that these rules are handy ones: Electron configurations list the orbitals from lower to higher energy. For example, when I show you the electron configuration for oxygen (1s²2s²2p⁴), this means that the 1s orbital is lowest in energy, followed ...

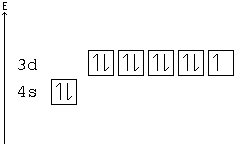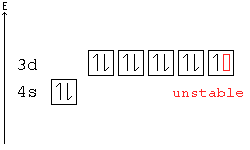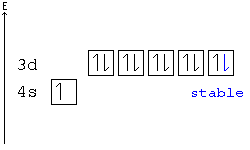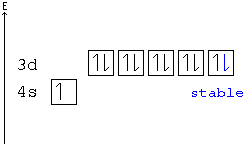





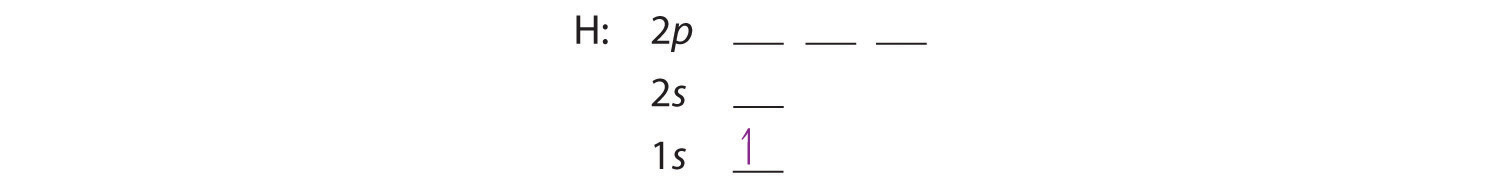



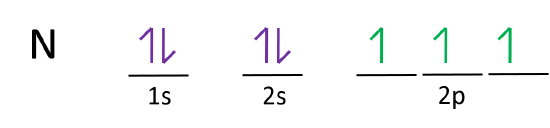

:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)






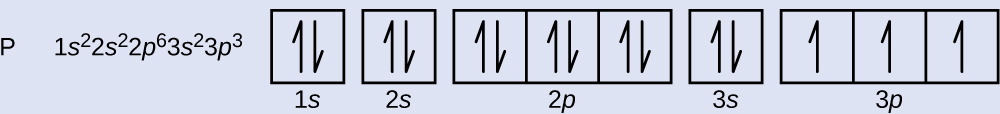




0 Response to "37 orbital filling diagram for calcium"
Post a Comment