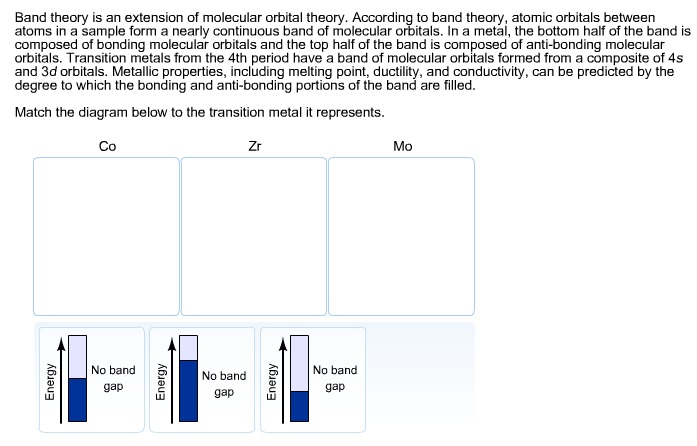
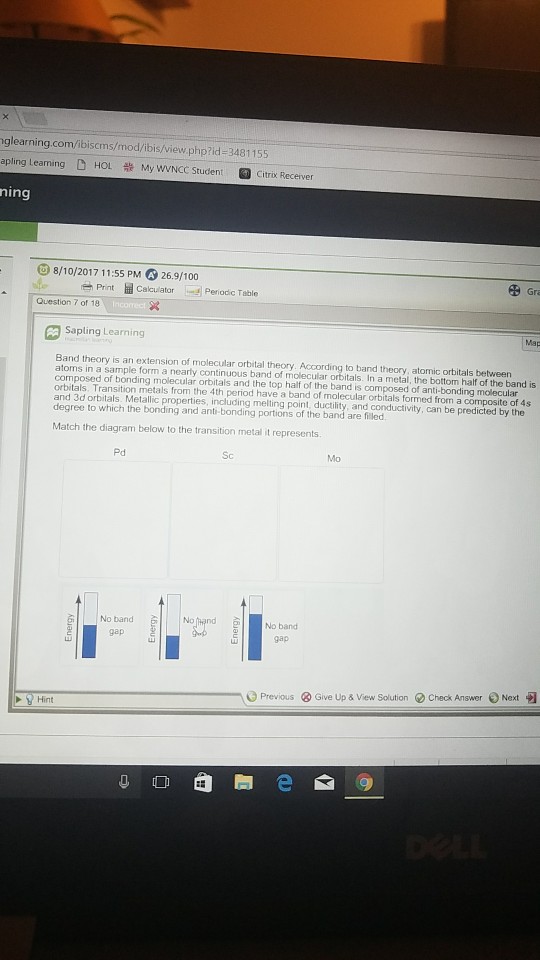
37 match the diagram below to the transition metal it represents.
The diagram represents one way an enzyme can be inhibited The diagram represents one of mendel's laws or principles of inheritance. One way to increase the volume of the gas in the balloon in the diagram above is to - Energy can be transformed from one form to another. the diagram shows one such process. Refer to the diagram to the right. the firm represented in the diagram makes PDF Chapter 24 Chemistry of Coordination Compounds • suggested in 1893 that metal ions have primary and secondary valences. ! Primary valence equals the metal's oxidation number ! Secondary valence is the number of atoms directly bonded to the metal (coordination number) Co(III) oxidation state Coordination # is 6 Cl-
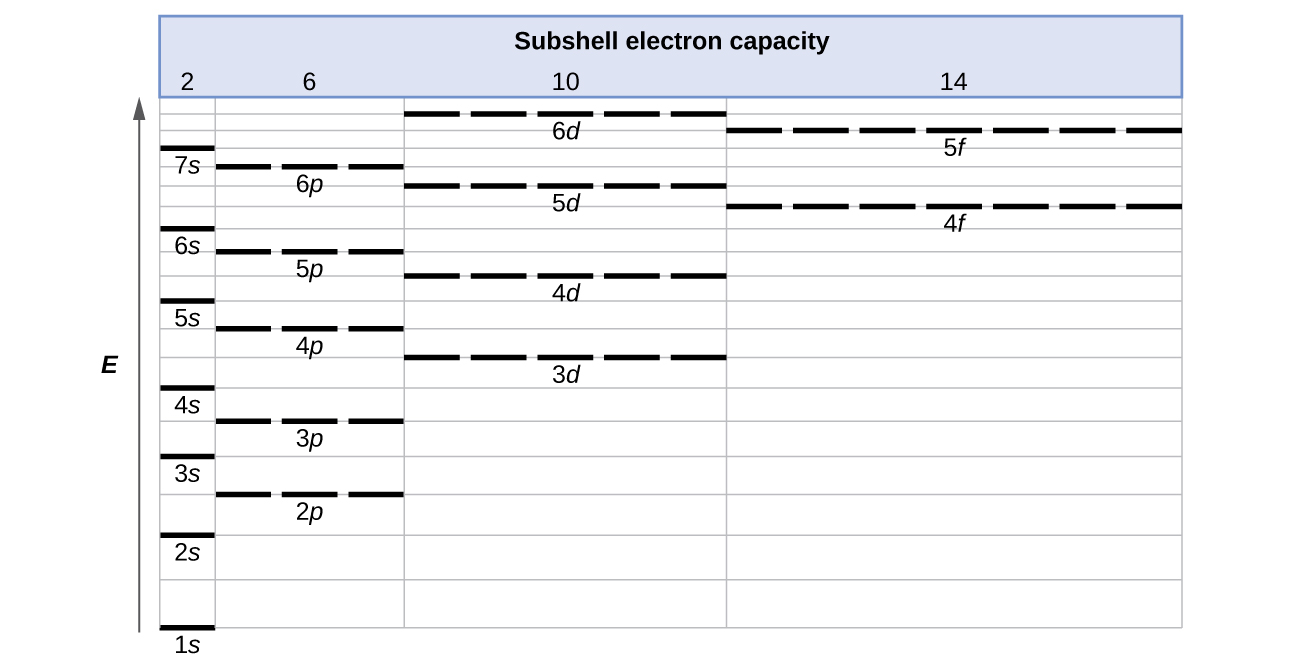
Electronic Structure of Atoms (Electron Configurations ... The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium.
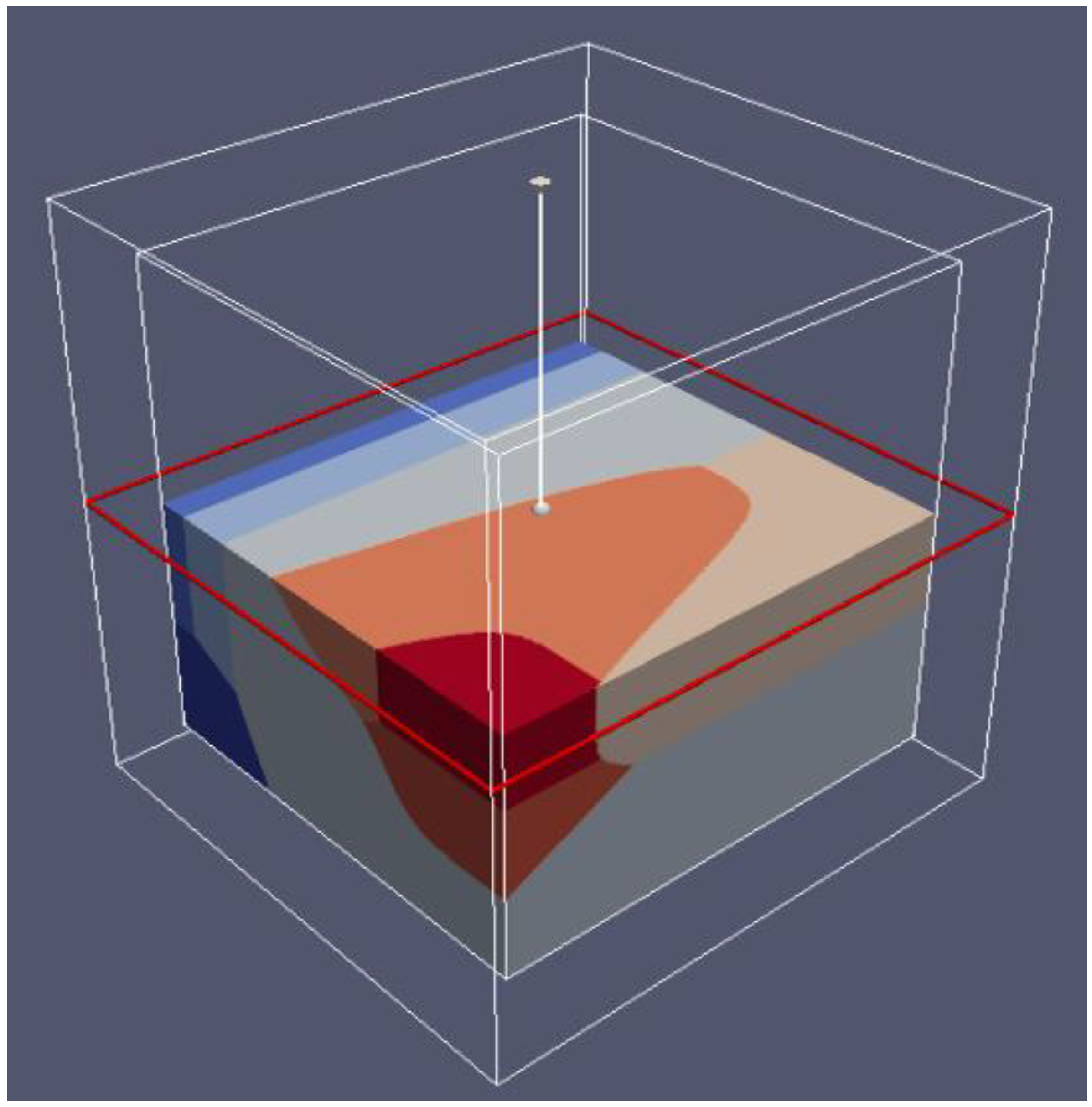
Match the diagram below to the transition metal it represents.
Solved Band theory is an extension of molecular orbital ... Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents. Solved Band theory is an extension of molecular ... - Chegg Transition metals from; Question: Band theory is an extension of molecular orbital theory According to band theory, atomic orbitals between atoms in a sample form a nearly continuous band of molecular orbitals. In a metal, the bottom half of the band is composed of bonding molecular orbitals and the top half of the band is composed of anti ... Solved 1. Based on the proposed reaction mechanism below ... Identify any reaction intermediates present in the mechanism. Step 1 slow Step 2 NO2 (g) + NO2 (g) → N2049) N,0.9) NO (g) + NO3 (9) NO (9) NO (g) + O2 (g) fast fast Step 3 Learning Target K.2: I can interpret an energy diagram to identify the number of mechanistic steps, the rate-limiting step, and the identities of any transition states ...
Match the diagram below to the transition metal it represents.. Match the diagram below to the transition metal it represents. The diagram below represents currents in a segment of an electric circuit For the following question, match the key event of meiosis with the stages listed below. Which of the following is true about the metal boxes pictured below List of Elements in the Transition Metal Group The transition metals are malleable (easily hammered into shape or bent). These metals tend to be very hard. Transition metals look shiny and metallic. Most transition metals are grayish or white (like iron or silver), but gold and copper have colors not seen in any other element on the periodic table. The transition metals, as a group, have ... PDF ANSWER. Series #2 - LSU The transition labeled "e". 4. Of the five separate electron transitions that have been labeled with letters in the energy-level diagram, which results in the production (or destruction) of the longest wavelength photon? ANSWER. The transition labeled "c". 5. Given that the "Hα" absorption line identified in the hydrogen spectrum ... Chem ch 3 smartworks hw: Flashcards - Quizlet Match the six colors with the appropriate part of the hydrogen energy-level diagram to indicate the color of each emission. F (starting at n = infinity) to C to D to A to E to B (ends at n =1). Using only the periodic table, arrange each set of particles by relative radius.
Part A Match each diagram to the atom or ion it represents ... Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. You did not open hints for this part. ANSWER: Part B What is the charge on the most stable ion of each of the following elements? Drag each item to the appropriate bin. You did not open hints for this part. Chem Exam #2 (Chem 101 Ch 3 hw) Flashcards - Quizlet Match. Gravity. Created by. maddiejb13. Terms in this set (70) ... A student draws the orbital diagram below for the 3d electrons in a V atom. What, if anything, is incorrect about the drawing? ... Which of the following electron configurations represent a transition metal atom? Answer Key: ATOM and PT Study Guide and ... - Google Search The chart below shows the percentages of elements in the Earth's crust. Excluding the "Other" category, what percentage of the Earth's crust is . a. alkali metals? 5.4%. b. alkaline-earth metals? 5.6%. INTERPRETING GRAPHICS . 19. Study the diagram below to determine the pattern of the images. Predict the missing image, and draw it ... FAASICPMS Section 1.2 - Whitman College The possible transitions shown in Figure 1-3 are specific to a particular element and such a diagram can be constructed for every metal. When energy (or a photon) corresponding to a specific transition is absorbed, the electronic state is changed (i.e. from 3s to 3p).
(a) Comparison of DSC results of the electrochemically ... The transition metal and Na layer thicknesses can be calculated from Equations (1) and (2) as mentioned below. Similar studies were performed by Li et al. [33] . ... Chapter 5 Concept Quiz Flashcards | Quizlet B. You can tell because the electron jumps up from 0 eV to 10.2 eV. The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. Which of the following transitions would result in the ... We see from the energy level diagram that the energy levels get closer together as #n# increases. This, the smallest energy and the longest wavelength is associated with the #n = 7 → n = 8# transition. I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead. MODULE #4 HW Flashcards | Quizlet Look at the free energy diagram for two distinct reactions, reaction 1 and reaction 2. Reaction 1: A -----> B Reaction 2: X -----> Y A. Both reactions are spontaneous, but reaction 1 is slower than reaction 2. B. The transition state for reaction 2 is easier to achieve, since the free energy of X is higher than the free energy of A. C.
PDF Final Exam Review 1.Electricity and Magnetism Name Free ... 37.On the diagram below, sketch at least four electric field lines with arrowheads that represent the electric field around a negatively charged conducting sphere. 38.Two oppositely charged parallel metal plates, 1.00 centimeter apart, exert a force with a magnitude of
d-metal complexes Practice Problems Answers Yet, since Cr is a first row transition metal, the ionic radius is still small enough that the coordination sphere can not expand to 7-coordinate (or higher) without introducing sizable steric repulsions. These combination of factors (and probably others) lead to the high incidence of 6-coordinate Cr 3+ complexes.
Match The Diagram Below To The Transition Metal It ... band theory match the diagram to the transition metal it match the diagram to the transition metal it represents what band represents the truest form of metal music which albums by metal mastering chemistry chapter 2 assignment flashcards match each diagram to the atom or ion it represents drag each item to the appropriate bin wiki figure 10 MLCT
HW #7 Flashcards - Quizlet The diagrams below show the same set of energy levels as in Parts A and B, but with a different set of electron transitions (notice that the arrows are now different). Assuming that these electron transitions were caused by the absorption of a photon, rank the atoms based on the energy of the absorbed photon, from highest to lowest.
DOC Class worksheet for Chapter 5: An Introduction to ... The diagram represents a schematic P-T phase diagram for the melting of a mineral. Write the melting reaction and express G in terms of the Gibbs free energy of the reactants and products. What is the sign or value of G at points A, B, and x in the figure? Explain.
PDF Name: Period: IPS Unit 8 Periodic Table Review Worksheet 11. An electron dot diagram uses the symbol of an element and dots to represent the (quarks/electrons) in the outer energy level. Atoms 1. have a mass number equal to are organized the sum of as elements in the are made up of 4. 5. 2. 6. and and and
Solved 1. Based on the proposed reaction mechanism below ... Identify any reaction intermediates present in the mechanism. Step 1 slow Step 2 NO2 (g) + NO2 (g) → N2049) N,0.9) NO (g) + NO3 (9) NO (9) NO (g) + O2 (g) fast fast Step 3 Learning Target K.2: I can interpret an energy diagram to identify the number of mechanistic steps, the rate-limiting step, and the identities of any transition states ...
Solved Band theory is an extension of molecular ... - Chegg Transition metals from; Question: Band theory is an extension of molecular orbital theory According to band theory, atomic orbitals between atoms in a sample form a nearly continuous band of molecular orbitals. In a metal, the bottom half of the band is composed of bonding molecular orbitals and the top half of the band is composed of anti ...
Solved Band theory is an extension of molecular orbital ... Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents.










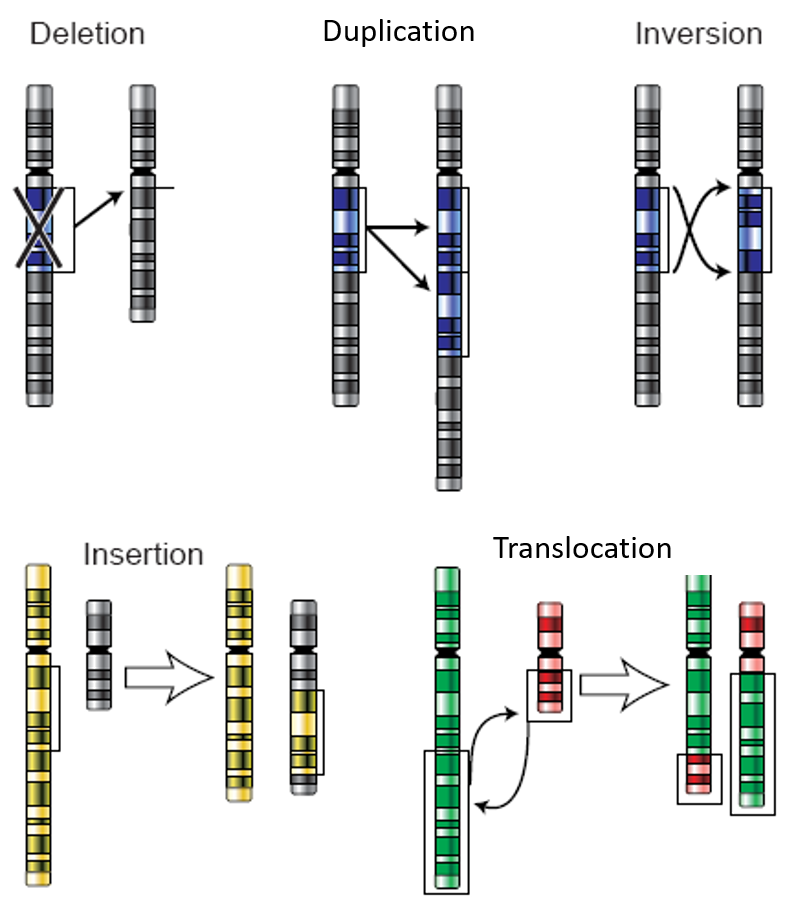
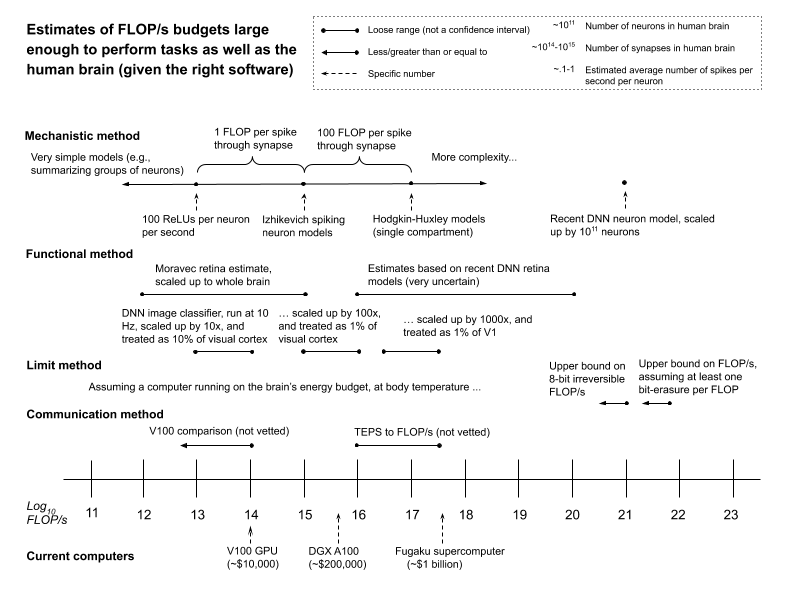
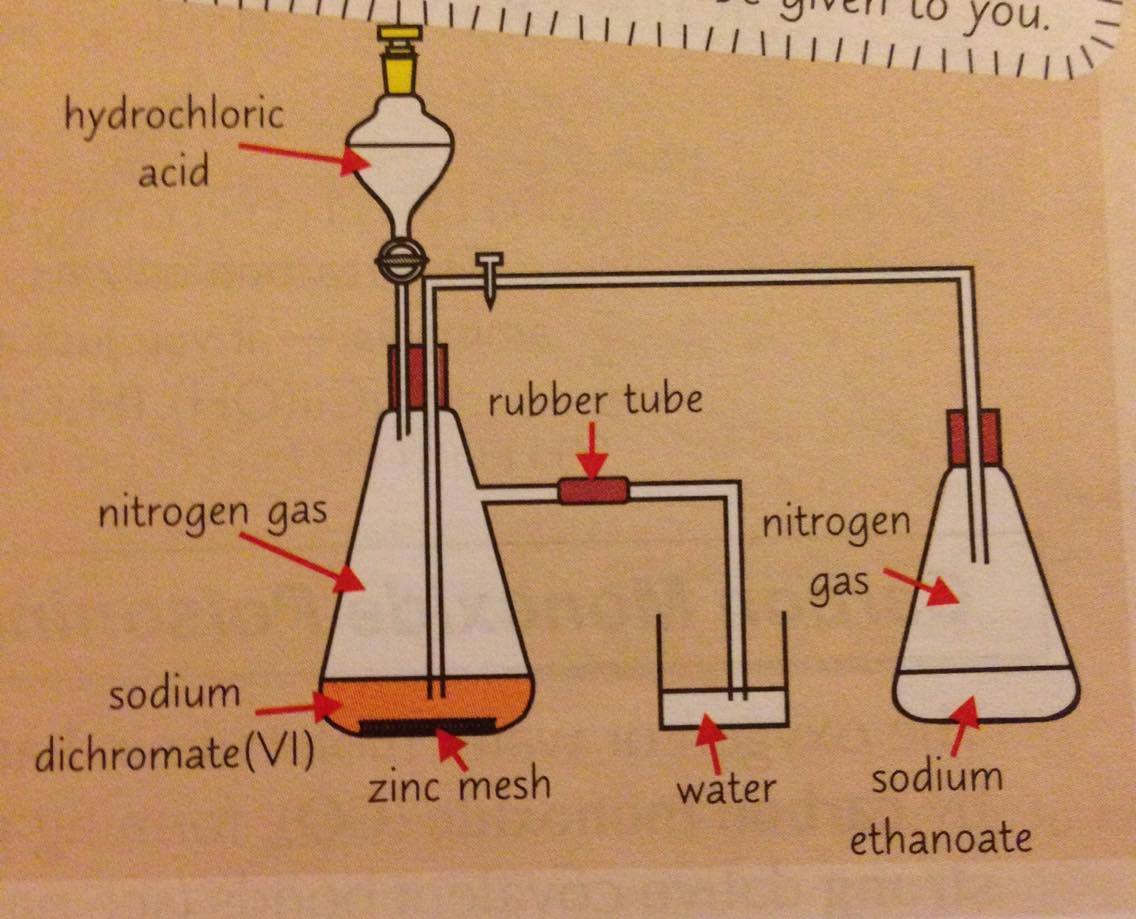
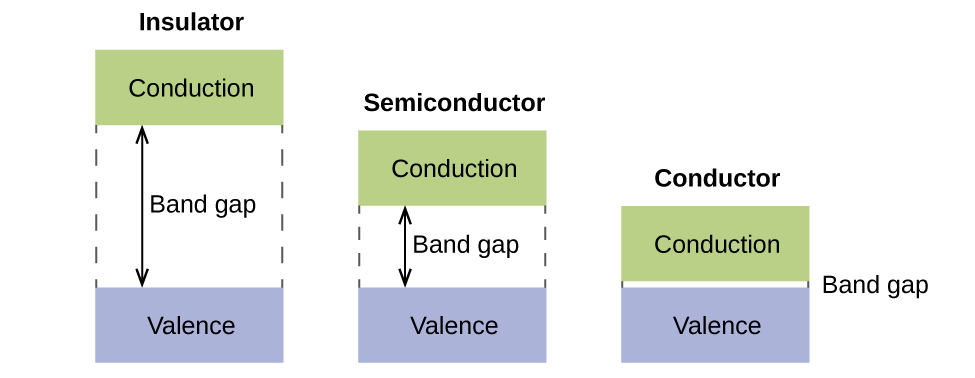







0 Response to "37 match the diagram below to the transition metal it represents."
Post a Comment