41 mo diagram for he2
which of species is diamagnetic? 1) H2- 2) H2+ 3) H2 4) He2+ 1) H2- 2) H2+ 3) H2 4) He2+. A species is said to be diamagnetic when it has all the paired electrons. Similarly if the species contain unpaired electron it is said to be paramagnetic. To know the magnetic character of molecules we can use MO diagram. When we draw MO diagram for dihydrogen anion ( H2-) we find one unpaired electron in ... Lecture 10: LCAO MO, Energy Level Diagrams for H2, He2 ... Lecture 10: LCAO MO, Energy Level Diagrams for H2, He2, Li2 Course Home Syllabus Calendar Readings ... And I am going to superscript it molecular orbital, and this upper one, to indicate that it's antibonding, has the asterisk. This is sigma star with the antibonding orbital that came from 1s, and it is a molecular orbital.
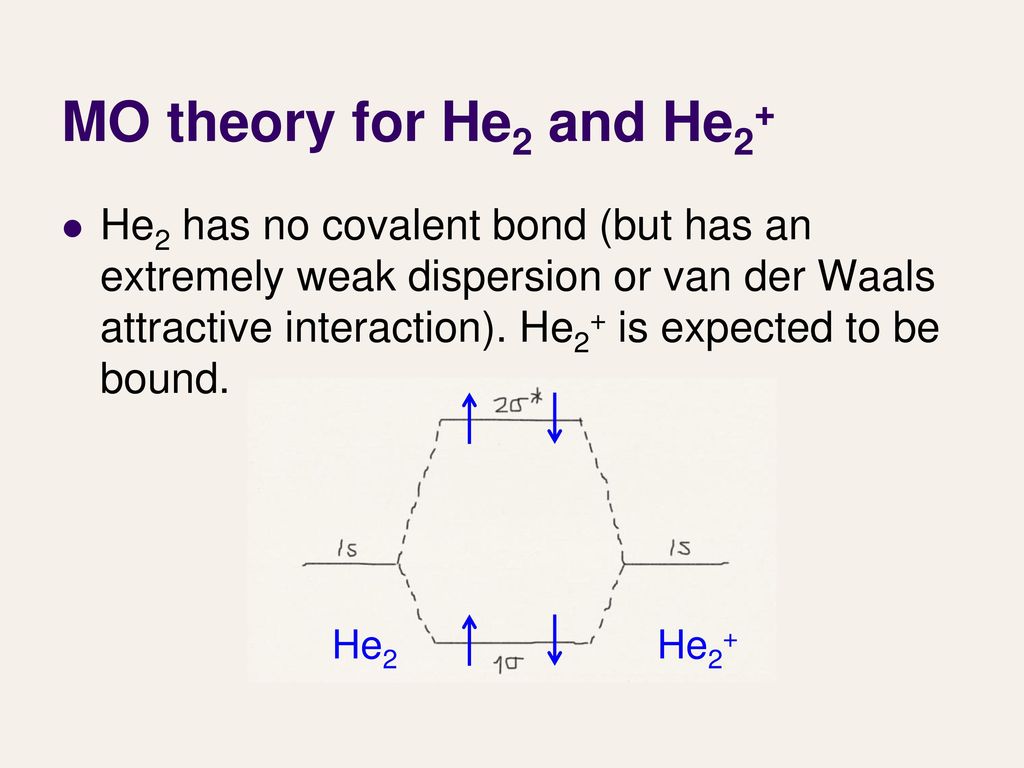
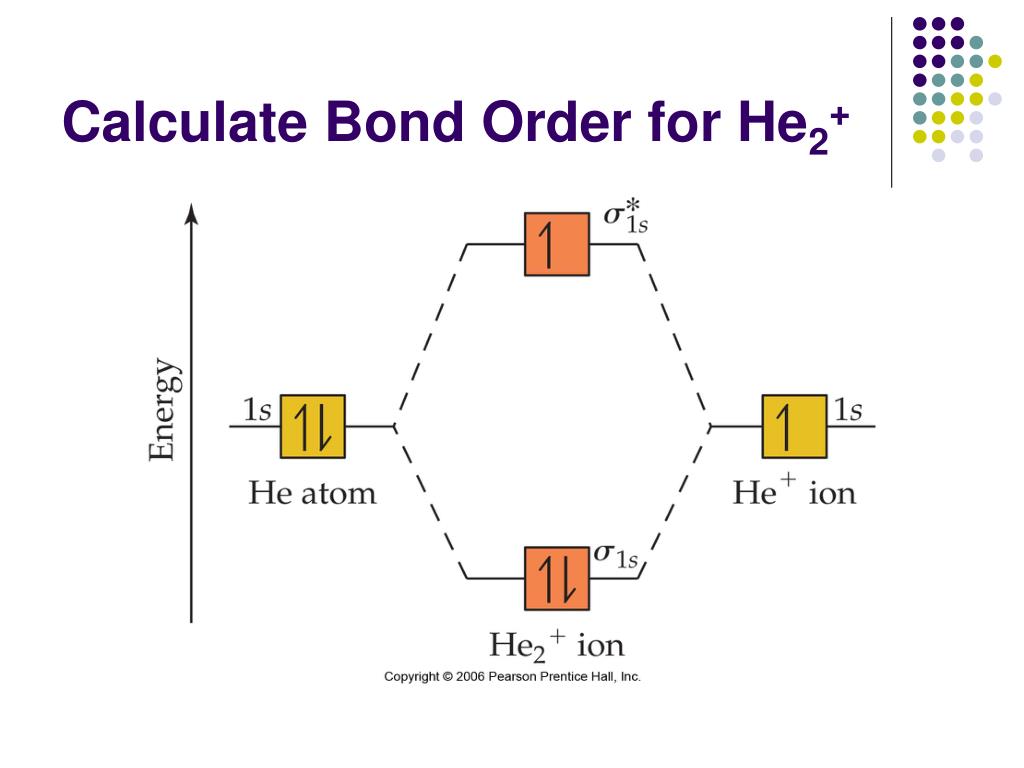
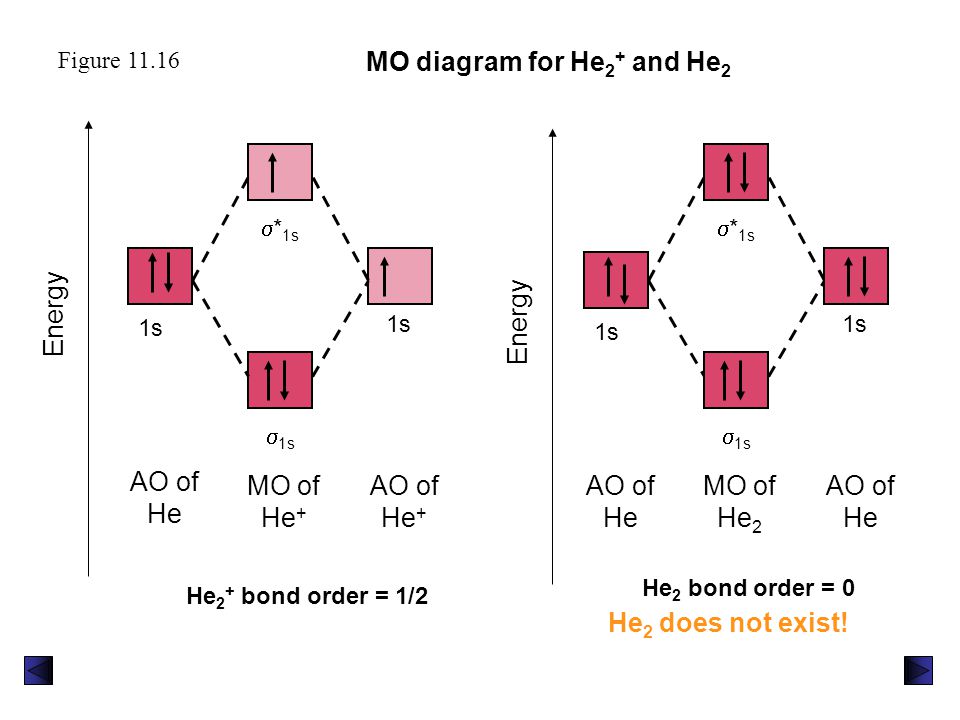
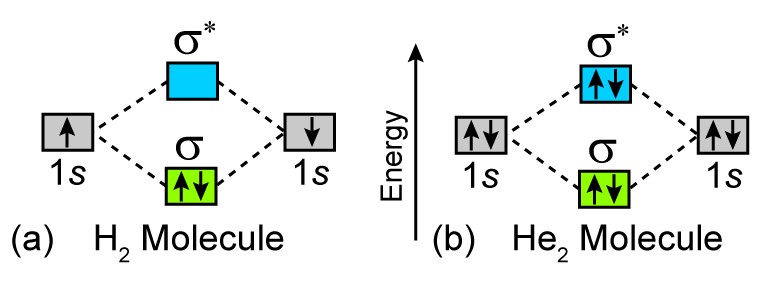
Molecular Orbital Diagram For He2+ - schematron.org The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
Mo diagram for he2
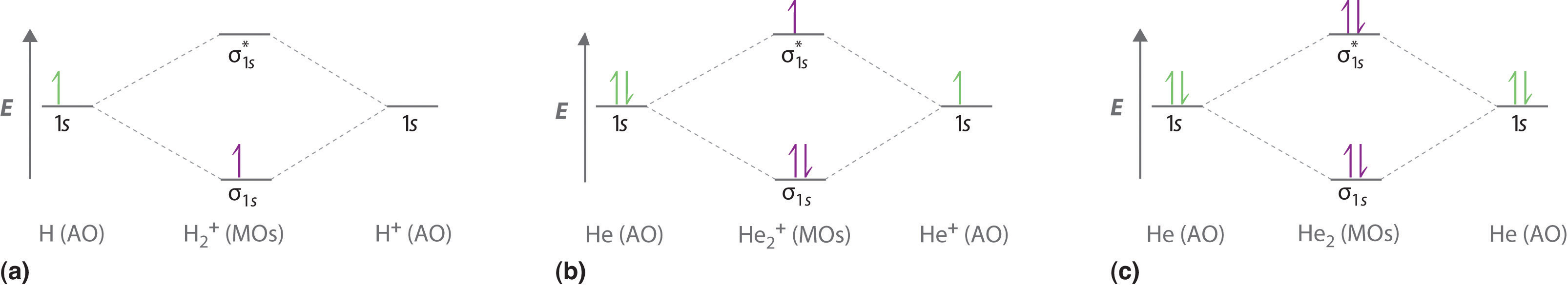
Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−. Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top 2 posts • Page 1 of 1 He2 2+ Molecular Orbital Diagram - schematron.org the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
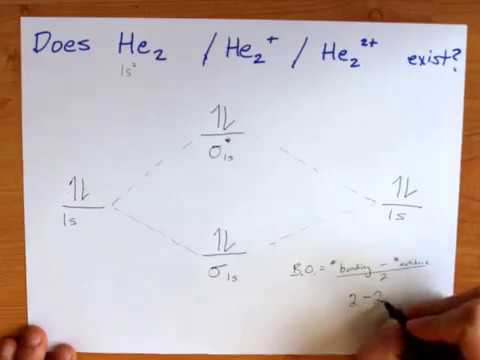
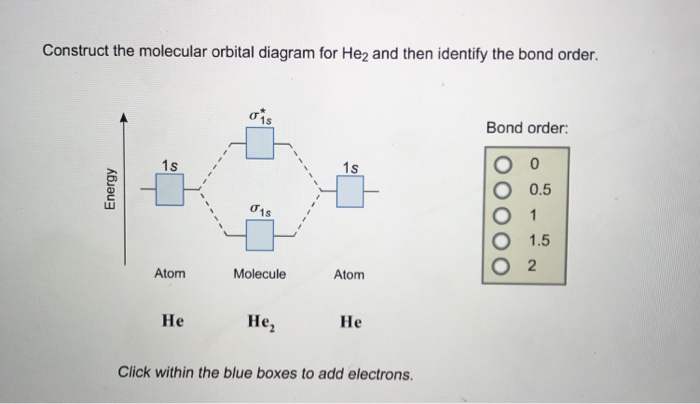
Mo diagram for he2. Molecular orbital correlation diagrams for He2, He2+, N2 ... The correlation diagrams for nitrogen and carbon monoxide and the first positive ions of each symmetry type are all similar in shape, especially for the innermost orbitals. Ionization potential curves plotted on the diagrams for the neutral systems are nearly parallel to the corresponding orbital energy curves. Solved Whether or not the diatomic He2, He2+, and He2 ... Whether or not the diatomic He2, He2+, and He2- exist (on the basis of MO diagram) In each case show the MO diagram and Bond order. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... Mo Diagram He2 Mo Diagram He2 Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. According to Molecular Orbital (MO) Theory, two atoms mix their orbitals to form one that is spread out over both atoms. The mixing of two.
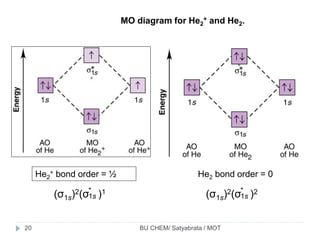
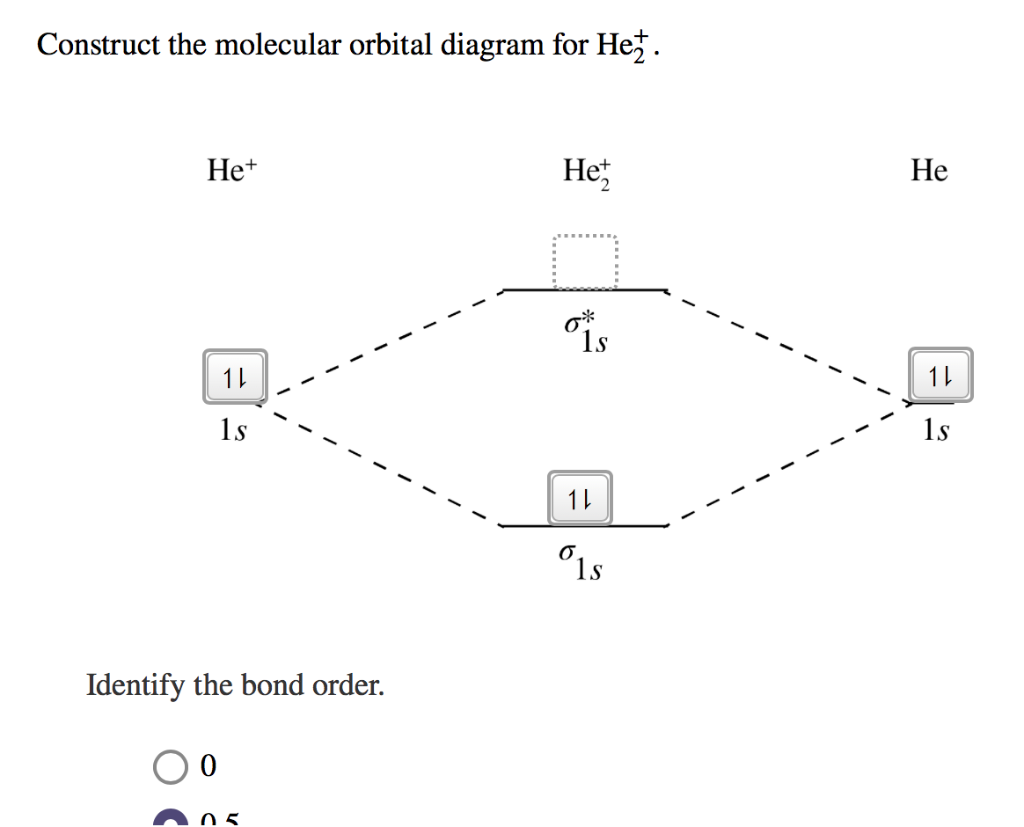
He2 2+ Molecular Orbital Diagram - Wiring Diagrams H−. Bond order = 1. 2 (electrons in bonding orbitals - electrons in antibonding orbitals) Draw a complete MO diagram for all the bonds in ethene.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. What is the bond order of He2+?a. 0b. ½c. 1d. 1 ½e. 2 FREE Expert Solution. We're being asked to determine the bond order of He2+. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Construct the molecular orbital diagram for he2 Construct the molecular orbital diagram for he2 Answer General guidance Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength of the molecule; If the bond order is more, the bond strength would also be more for that molecule. . Fundamentals Solved Draw the molecular orbital (MO) electron diagram ... Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Question: Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion.
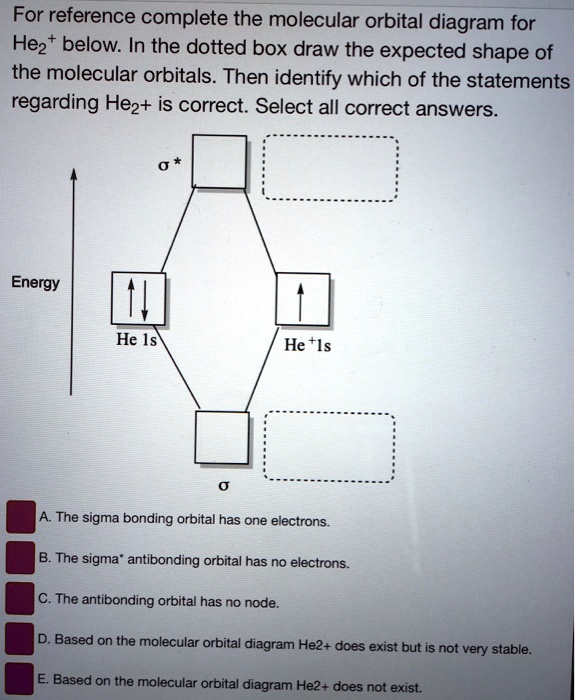
Energy-level diagram for the He2+ ion.Whic... | Clutch Prep FREE Expert Solution Recall: The bond order determines the stability of a molecule based on it's molecular orbital diagram. 86% (85 ratings) View Complete Written Solution Problem Details Energy-level diagram for the He2+ ion. Which electrons in this diagram contribute to the stability of the He2+ ion? Mo Diagram For He2 albugerrl on slatatphorec Danielle Soto on Mo Diagram For He2 albugerrl. Oct 6, 2014 — MO diagram of H2: In the case of H2, both electrons are in the σ1s orbital. MO diagram of He2: Electron configuration of He2: There is in. Construct the molecular orbital diagram for he2 - Soetrust Spanish. Tech. World Languages. Soetrust. Misc. Construct the molecular orbital diagram for he2. by soetrust February 25, 2022 Leave a reply 1. SOMEONE ASKED. Construct the molecular orbital diagram for he2. 7.7 Molecular Orbital Theory - Chemistry Fundamentals Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. Figure 7.7.12. This shows the MO diagrams for each homonuclear diatomic molecule in the second period.
What is the MOED of He2 molecule class 11 chemistry CBSE And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0 So, the bond comes out to be zero, therefore the H e 2 molecule is unstable and does not exist.
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Why He2 molecule does not exist? Explain by MOT. Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist.
PDF Mo diagram for he2 Mo diagram for he2 Construct an mo diagram for the he2+ ion. Mo diagram for he2 2+. Mo diagram for he2+ ion. Mo energy level diagram for he2. Although molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms.
Answered: Draw the molecular orbital (MO)… | bartleby Draw the molecular orbital (MO) electron diagram for the He2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons.
Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
PDF Mo diagram for he2 Mo diagram for he2 Mo energy level diagram for he2. Mo diagram for he2+ ion. Mo diagram for he2 2+. Construct an mo diagram for the he2+ ion. 1Molecular Theory Orbital: General Vision of Claro11: 32mins2Molecular Theory Orbital and its Postulates 15: 00mins3LCAO Model, Conditions of OBITALS ATMICAS15: 00mins4Energy Non-viable Diagram for Molecular Orbitals 15: 00mins5Entronic Configuration ...
Molecular Orbital Diagram of H2, He2, Li2 and Be2 ... 0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo...
__FULL__ Mo Diagram For He2 on uscomporthu The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the .... Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding ( 1σ) and ....
Molecular Orbital Diagram For He2 The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
He2 2+ Molecular Orbital Diagram - schematron.org the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top 2 posts • Page 1 of 1
Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.




















0 Response to "41 mo diagram for he2"
Post a Comment