37 no2- molecular orbital diagram
Academia.edu is a platform for academics to share research papers. PPN4T-2F has a large optical bandgap of 2.15 eV and a deep the highest occupied molecular orbital level of -5.43 eV. Solar cells based on PPN4T-2F and a …
Answer (1 of 2): This image shows the molecular orbitals of nitric oxide and the types of bonds present.
No2- molecular orbital diagram
Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. sp 2 hybridisation. The electronic configuration of carbon (Z = 6) in the excited state is. In this type of hybridization one- s and two P-orbitals of the valence shell of carbon atom take part in hybridization go give three new sp 2 hybrid orbitals. These sp 2 hybrid orbitals lie in a plane and are directed towards the corners of an equilateral triangle with a carbon atom in the centre. This problem has been solved! See the answer. See the answer See the answer done loading. NO2+ molecular orbital diagram. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating)
No2- molecular orbital diagram. Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ... Sketch the MO diagram for C4H6 (1,3-butadiene, CH2=CH–CH=CH2). Page 3. 3. 3. Assume light is absorbed by NO2. − to create the excited molecule (NO2.5 pages When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals. D) Electrons placed in antibonding orbitals stabilize the ion/molecule. E) All of the above are true. Associate membership to the IDM is for up-and-coming researchers fully committed to conducting their research in the IDM, who fulfil certain criteria, for 3-year terms, which are renewable.
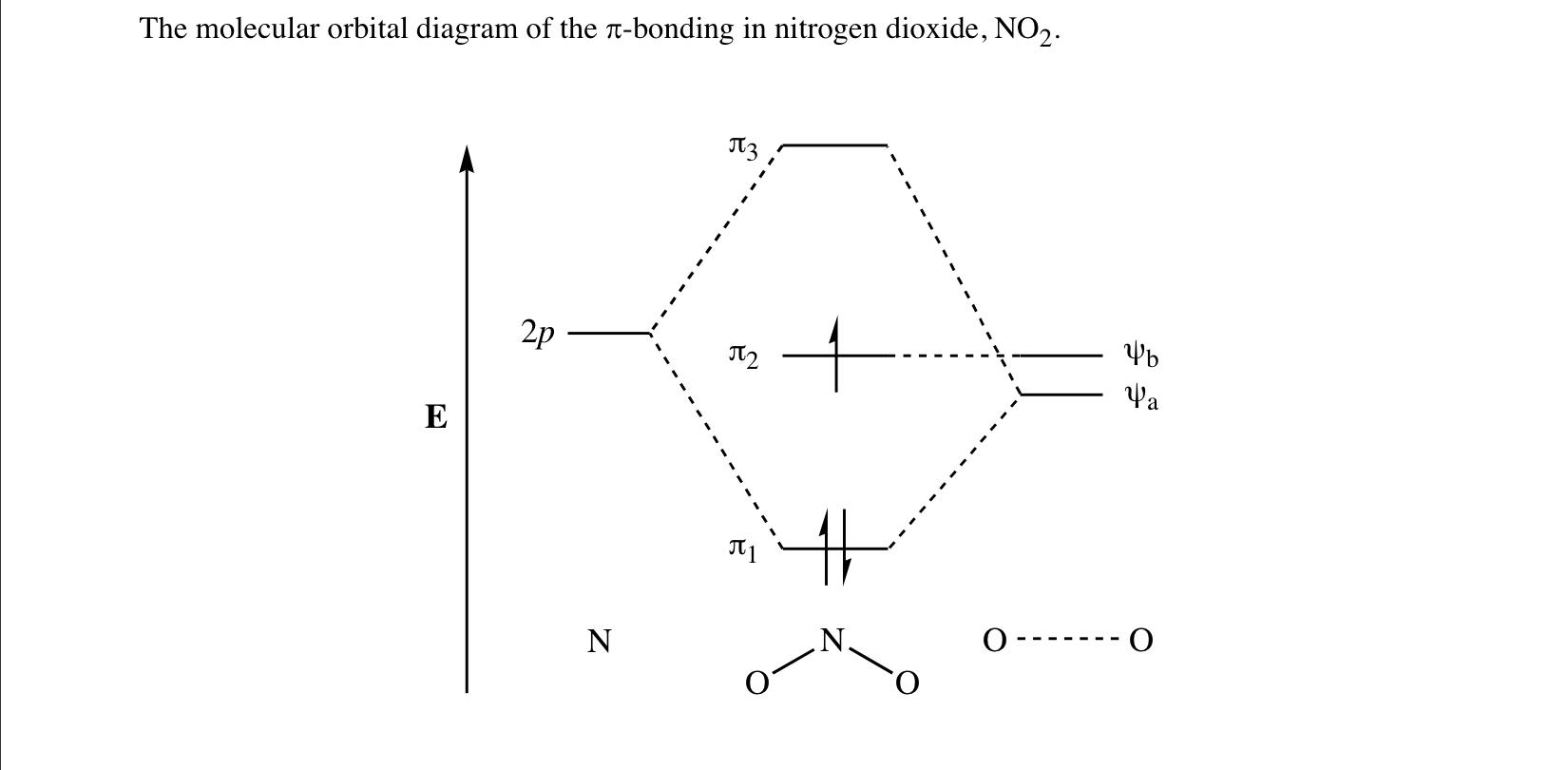
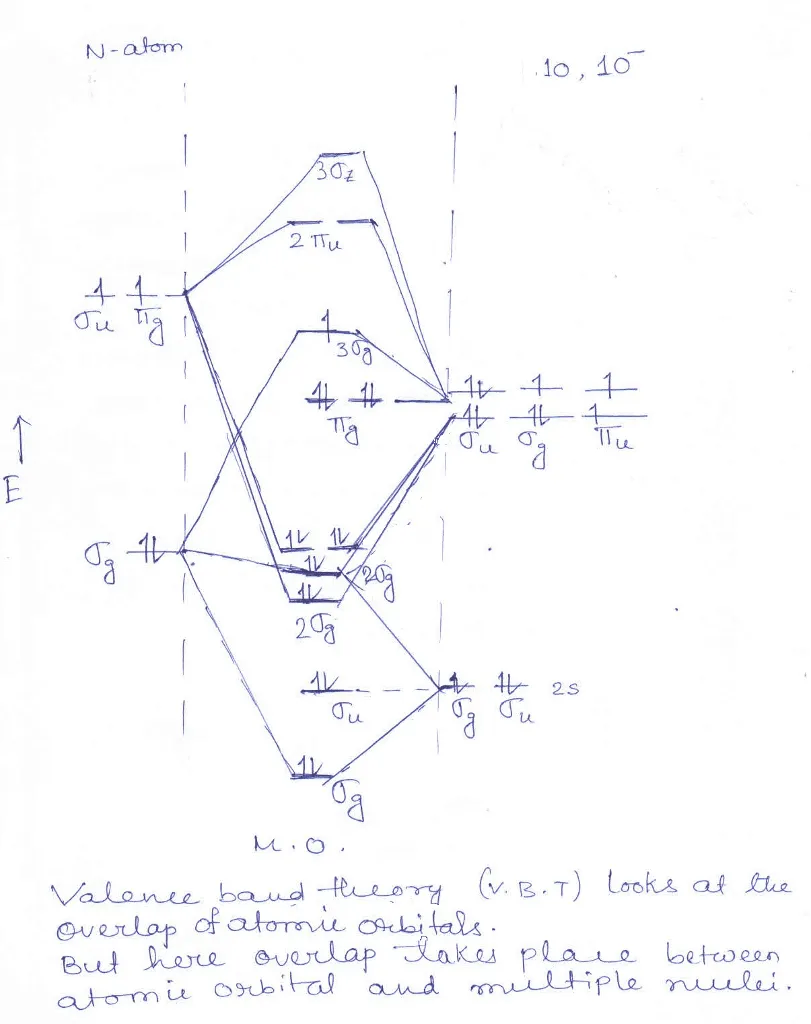
Oct 14, 2019 — Bond order = (No. of bonding electrons - No. · BO = (Nb - Na) / 2 · Now according to Molecular Orbital Theory…. · Let's start with NO first · Follow Hund's rule and ...1 answer · 4 votes: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite ...How do you find the bond order of NO2? - Quora3 answersAug 20, 2015What is the order of the bond lengths for NO2+ ...6 answersJun 29, 2017NO, NO+ and NO-. Using the molecular orbital theory ...5 answersJun 9, 2018What is the bond order for NO2+? - Quora2 answersOct 30, 2017More results from www.quora.com You've seen the molecular orbital (MO) diagram of CO2: CO2 and NO+ 2 are isoelectronic and thus have the same electron configuration. Thus, simply add one or two electrons into the 2b3u and 2b2u to get NO2 and NO− 2, respectively. Nitrogen atom has 2p atomic orbitals lower by 2.52 eV, and 2s atomic orbitals lower by 6.13 eV than with carbon atom. solucionario quimica de raymond chang 12 edicion . 697 Pages. solucionario quimica de raymond chang 12 edicion Consider the molecular orbital energy level diagrams for O2 and NO2. Which of the following is true? I. Both molecules are paramagnetic. II. The bond strength of O2 is greater than the bond strength of NO. III. NO is an example of a homonuclear diatomic molecule. IV. The ionization energy of NO is smaller than the ionization energy of NO^+. A ...
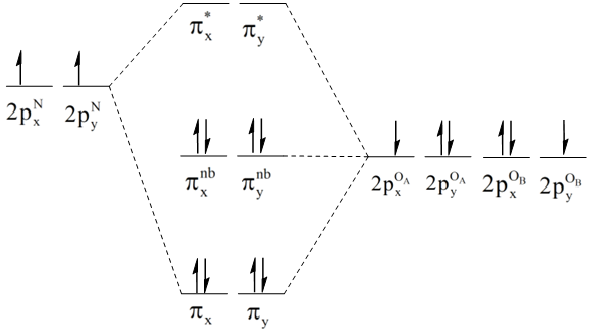
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... In both molecules the pi symmetry molecular orbitals are the same. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. Perpendicular to these in the yz plane, the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Here is the full molecular orbital diagram for N 2. 9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis: NEET 2020 The Solid State. 10. Find out the solubility of N i ( O H) 2 in 0.1 M NaOH. Given that the ionic product of N i ( O H) 2 is 2 × 10 − 15. NEET 2020 Equilibrium. Photoelectron spectroscopy applies the principle of the photoelectric effect to study orbital energies of atoms and molecules. High-energy radiation (usually UV or x-ray) is absorbed by a sample and an electron is ejected. The orbital energy can be calculated from the known energy of the radiation and the measured energy of the electron lost.
Jan 24, 2022 · (a) Schematic diagram of a single-molecule junction formed by molecule 2 between gold electrodes. (b) Blatter radicals 1 and 2 with para and meta connection points and non-radical analogue 3, tight-binding (TB) spin-up (↑) and spin-down (↓) molecular orbitals for Blatter radical core with para (c) and meta (d) connectivities.
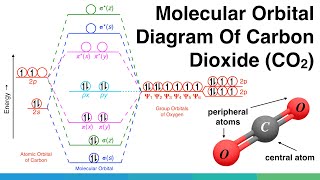
No2 Molecular orbital Diagram. molecular orbital theory structure of no2 pound the electron population of this orbital is see table vi 0 53 on the n atom 0 16 2s 0 37 2pz 0 24 on each o atom 0 24 pz experimentally oxides and oxyions of the non metals part ii c02 and no2 of the chemical society 1962 2873 2880 collects values for the partition of unpaired electron density among n and o atomic ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Feb 03, 2022 · 10. According to the molecular orbital energy level diagram O2- The key level of is _. 11. Compare the key angle size ( fill >,<, or =), And explain why : OF2 ____ OH2 , reason _____. AsF3_____AsH3, reason . 12. NO2+.NO2.NO2- The order of bond angle is ( From smallest to largest ). 13.
Solved Consider the following molecular orbital diagram for | Chegg.com. Science. Chemistry. Chemistry questions and answers. Consider the following molecular orbital diagram for NO2 : 2p ппы 41 = 2p Onb Energy 2s 46 # # 2s - 2s 2s 2s + 2s # N NOŻ 02 2 x 0 1. Please answer the following questions to ensure the MO diagram above is understood.
The molecular orbital diagram (MOT) is useful to predict bond order, bond strength, bond energy, stretching frequency, and bond length. Bond order has direct ...1 answer · Top answer: In a given molecule, one nitrogen atom and two oxygen atoms are present. The electronic configuration of N...
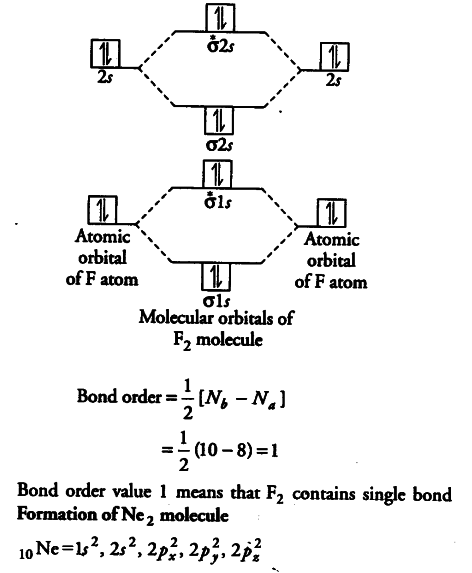
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Answer (1 of 5): NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 valence electrons: Same as NO+ but add (Pi*2px,Pi*2py)^1 NO- has 12 valence electrons: Same as NO but change ^1 at the end to ^2. Your MO diagram for NO should look like this: O is m...
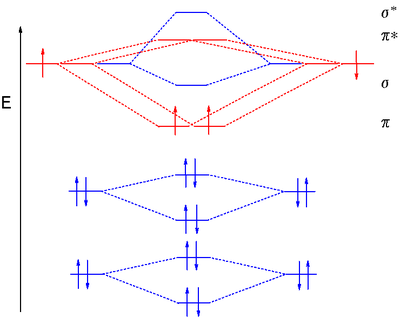
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
Academia.edu is a platform for academics to share research papers.
The Nitrogen atom in the Lewis structure for NO 2 is the least electronegative atom and passes at the center of the structure. Molecular Geometry and Bond Angles of NO 2. Since the Nitrogen Dioxide (NO 2) has an extra electron in a nitrogen atom orbital, it will result in a higher degree of repulsions. However, if we consider one lone electron ...
According to Electronic Structure of NO2 Studied by Photoelectron and Vacuum-uv Spectroscopy and Gaussian Orbital Calculations J. Chem. Phys. 53, 705 (1970) : The highest molecular orbital $4a_1$ is occupied by the 1 unpaired electron. The electron population of this orbital is (see table VI): 0.53 on the N atom (0.16 2s, 0.37 2pz)
We have an AX2E1 notation. VSEPR chart: We can see that NO2 has a bent molecular geometry and the angle is around 120 degrees. But here we have some exceptions. In NO2, we have 2 Bond Pairs and 1 lone electron. If we look at the nitrite ion NO2-, we have 2 Bond Pairs and 1 Lone pair of electrons.
Feb 11, 2020 · The orientation of the leaving group with respect to the anion does not matter at that point, so long as the C–LG antibonding orbital is aligned with the pi system. That’s because the pair of electrons from the breaking C-H bond have to donate into the antibonding orbital of the C-X sigma * bond . That isn’t the case in the E1cb.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
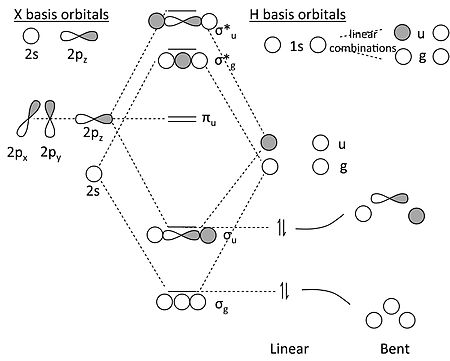
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
N2O4 Molecular Orbital (MO) Diagram A molecular orbital (MO) diagram explains the chemical bonding in molecules by energy level diagrams. They were proposed by Robert S. Mulliken and Friedrich Hund in 1928. As we know N2O4 molecule is a dimer of the NO2 molecule, hence we'll discuss molecular orbital diagram of NO2 molecule first.
Molecular orbitals in NO 2 Will the molecule be linear or bent? Click on a color picture to watch the geometry change from linear to bent. 2흅u: 2b 1 6a 1 : 1흅g: 1a 2 4b 2 : 1흅u: 1b 1 5a 1: Movies on this page were created as linear combinations of atomic orbitals by George Lisensky, Beloit College.
Hybridization of NO2 (Nitrogen Dioxide) NO 2 involves an sp 2 type of hybridization. The most simple way to determine the hybridization of NO 2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair.
For information on South Africa's response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal.
The energy of σ 2 p z molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behavior of the following species: ...
NCERT Exemplar Solutions Class 11 Chemistry Chapter 4 – Free PDF Download. NCERT Exemplar Chemistry Class 11 Chapter 4 Chemical Bonding and Molecular Structure is provided here to help students develop a clear approach to chemical bonding. Chemical Bonding and Molecular Structure is a very important topic that lays the foundation for all your future studies.
Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
This problem has been solved! See the answer. See the answer See the answer done loading. NO2+ molecular orbital diagram. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating)
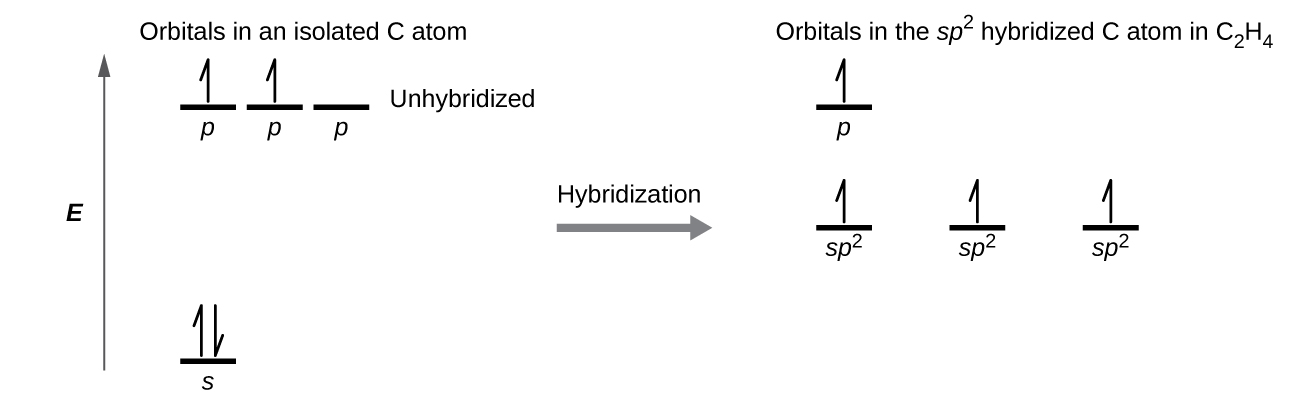
sp 2 hybridisation. The electronic configuration of carbon (Z = 6) in the excited state is. In this type of hybridization one- s and two P-orbitals of the valence shell of carbon atom take part in hybridization go give three new sp 2 hybrid orbitals. These sp 2 hybrid orbitals lie in a plane and are directed towards the corners of an equilateral triangle with a carbon atom in the centre.
Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule.



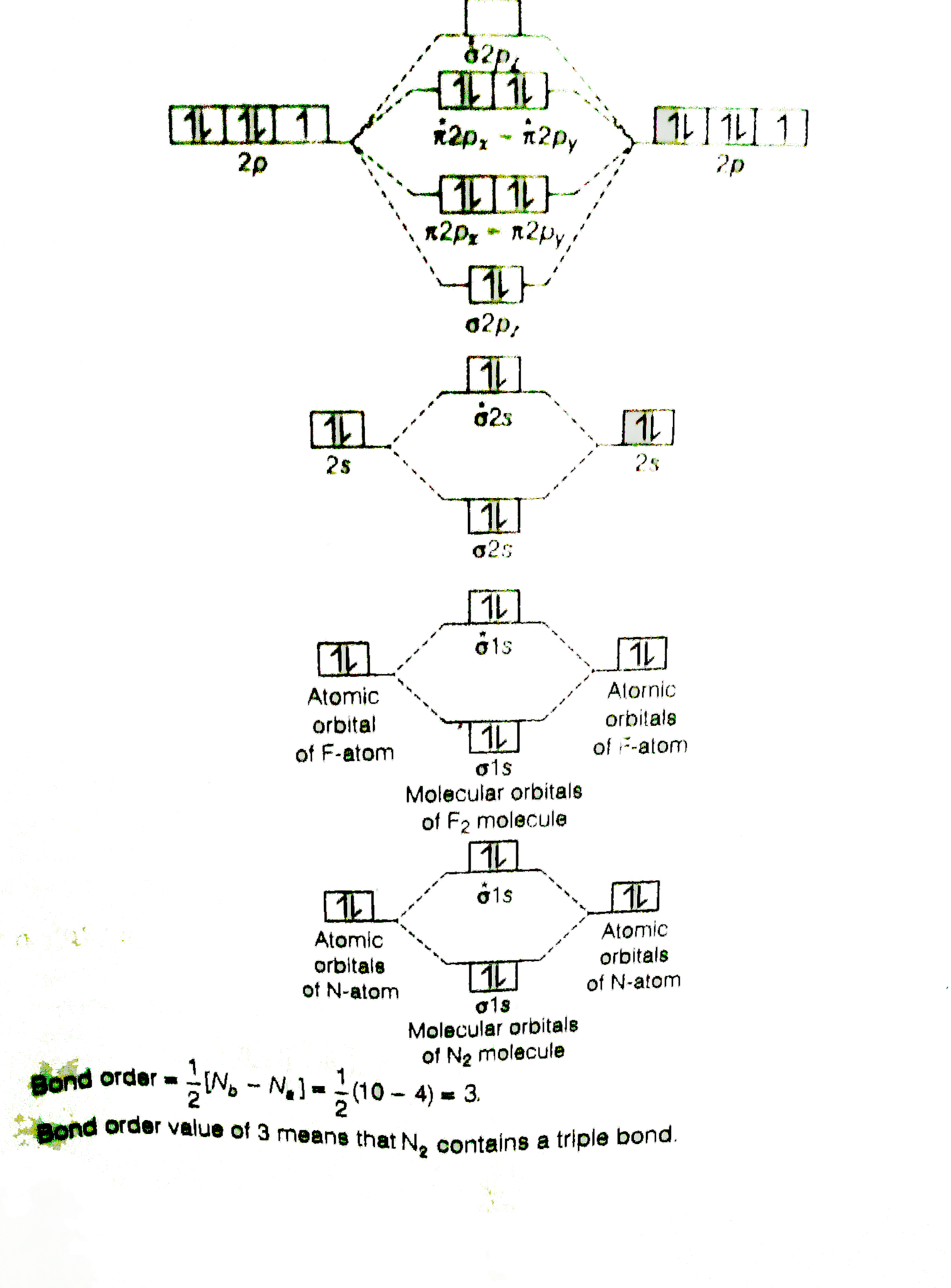






![Carbo -[3]oxocarbon and its isomers: evaluation of the ...](https://pubs.rsc.org/image/article/2008/CP/b718817j/b718817j-f5.gif)
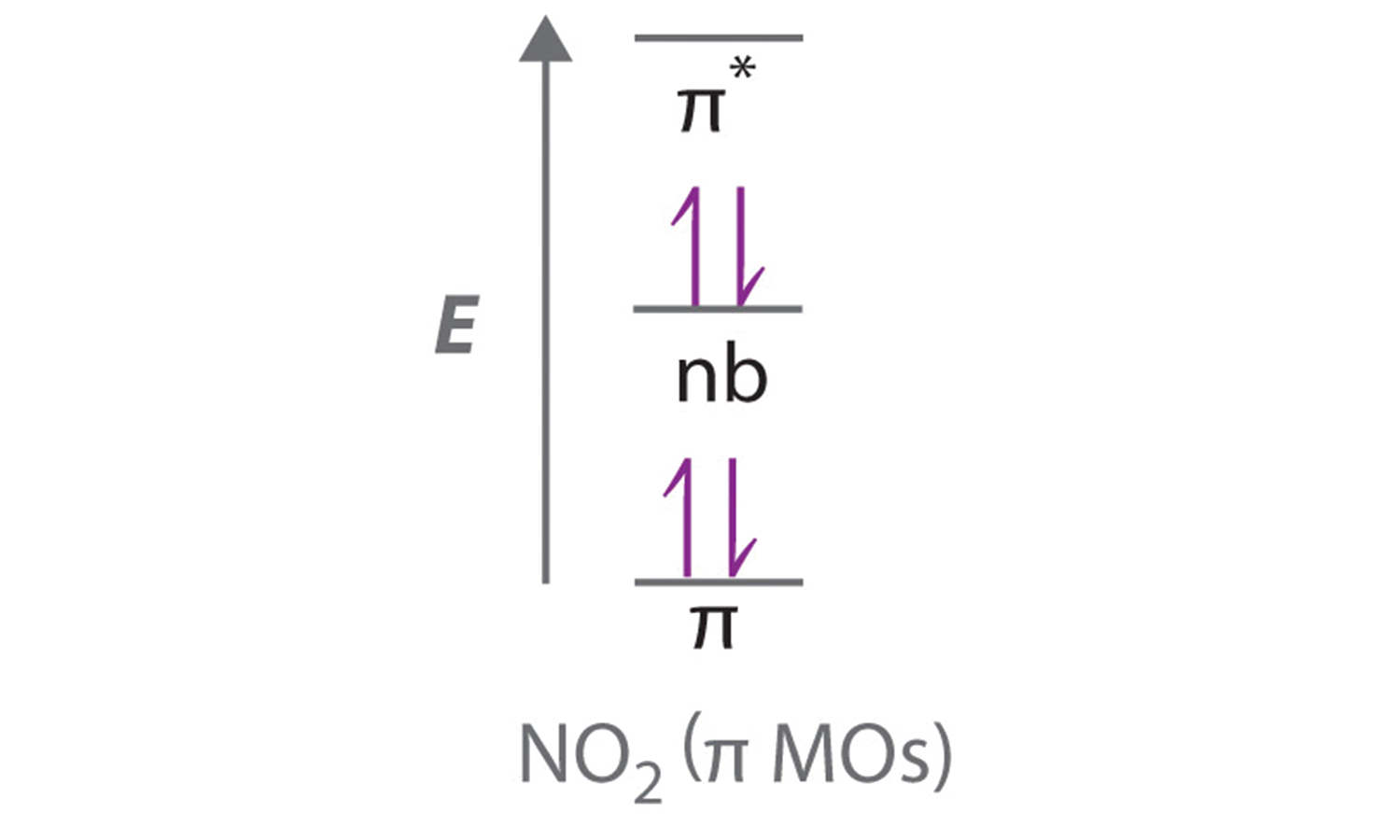





0 Response to "37 no2- molecular orbital diagram"
Post a Comment