37 methane molecular orbital diagram
Molecular formula = C 2 H 6 Molecular mass = 30 Empirical formula = CH 3 Empirical formula mass = 15 State: Gas at room temperature. Occurrence: Ethane occurs along with methane in natural gas and gases from oil-wells. It is also present in coal gas in very small quantity. Abstract. Orbital electron density distributions and momentum distributions of both core and valence space of methane are studied quantum mechanically, using RHF/TZVP and B3LYP/TZVP models, respectively. The three molecular orbitals (MOs) of methane in the ground electronic state (X 1 A 1 ), namely, the core MO, 1 a1 and the valence MOs, i.e. 2 a1 and three-fold energy degenerate MOs 1 t2, are studied in both coordinate space and momentum space.
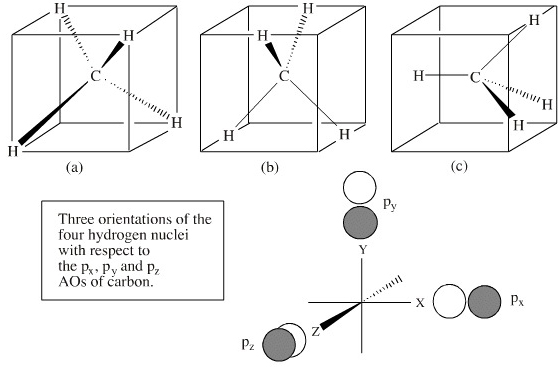
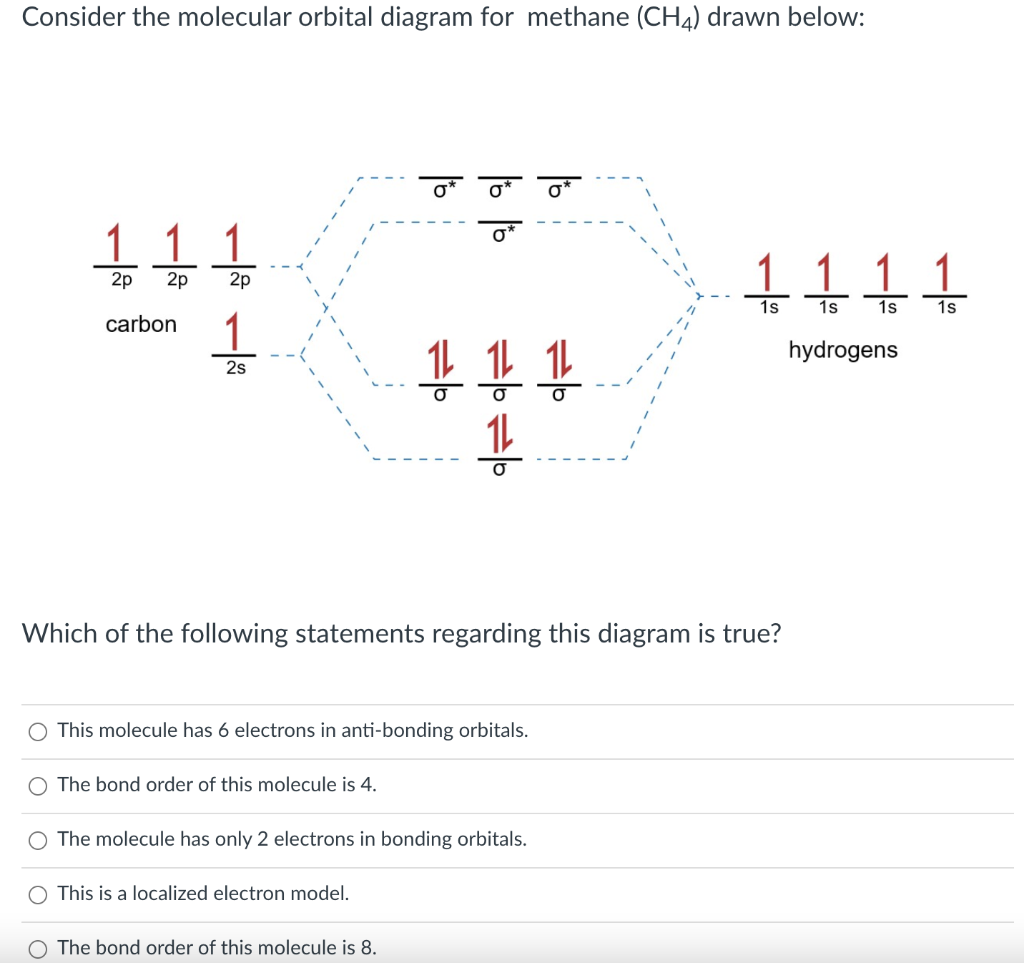
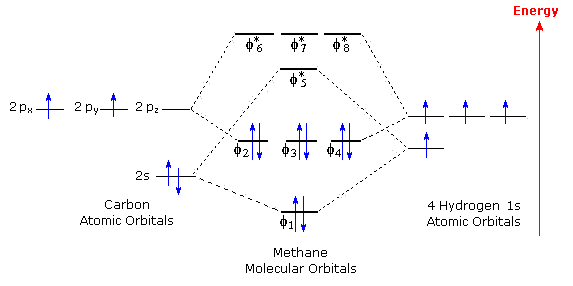
Methane is a tetrahedral molecule with four equivalent C-H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms.

Methane molecular orbital diagram
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons. The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The two carbon atoms bond by merging their remaining sp 3 hybrid orbitals end-to-end to make a new molecular orbital. The bond formed by this end-to-end overlap is called a sigma bond. The bonds between the carbons and hydrogens are also sigma ... • The carbon atom has one 2s orbital and three 2p orbitals • Each hydrogen atom has one 1s orbital • One can write the solution for the methane molecule as a linear combination of all available orbitals r c r r c s r c px r c py r c pz r j j s j 5 2 6 2 7 2 8 2 4 1
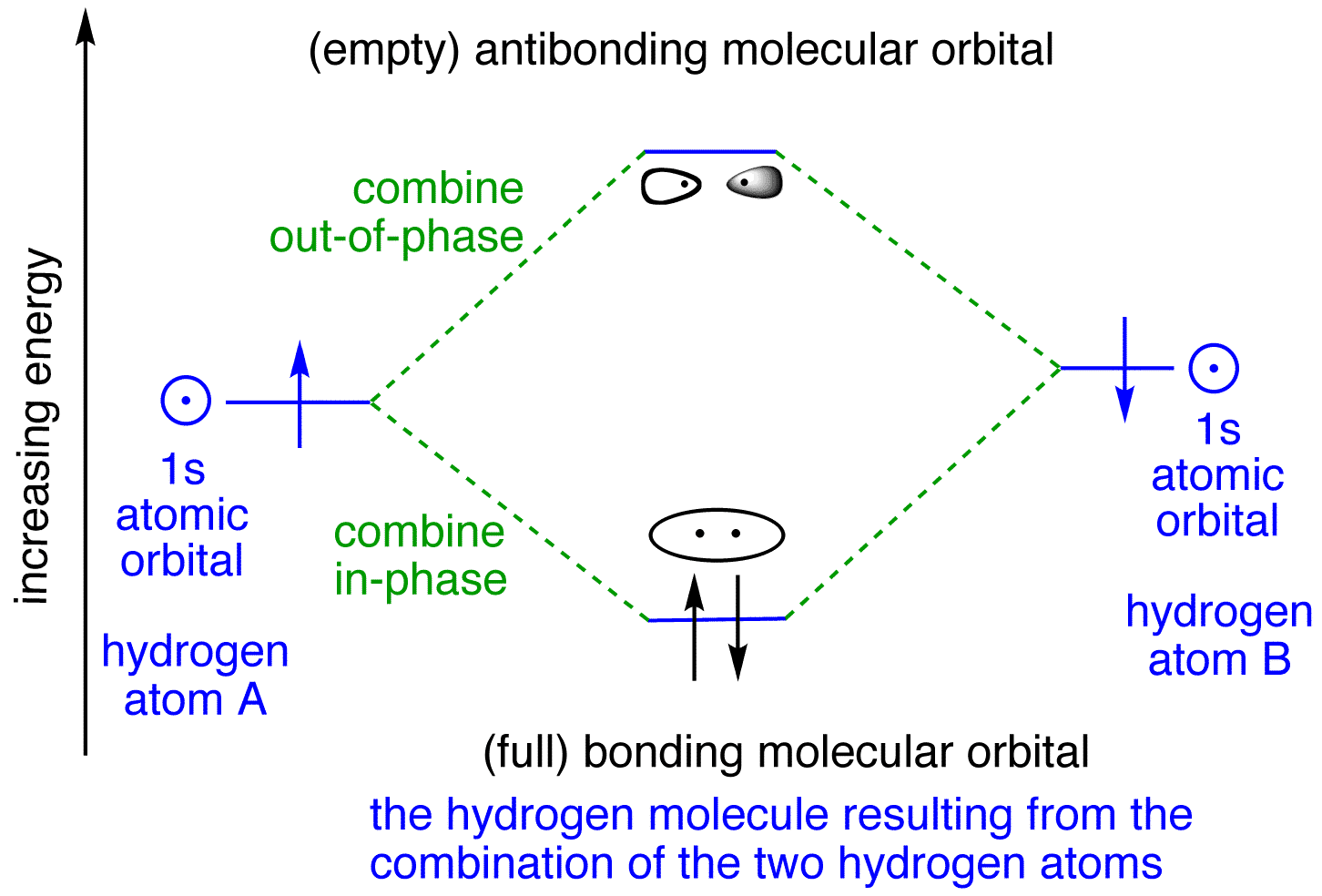
Methane molecular orbital diagram. Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle Home > Chemistry Article > CH4 lewis structure and its molecular geometry Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray. Hybrid atomic orbitals are shown in blue and yellow. Atomic p orbitals are shown in red and green. Greyscale Conventions: Hybrid orbitals are shown in grey. Unhybridized atomic orbitals are shown in reddish-grey. Forming Molecular Orbitals. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. Consider the H 2 molecule, for example. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1s atomic orbitals that come together to form this molecule. Another orbital is formed by subtracting one of these functions from the other, as shown in the figure below. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO’s: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom’s VB hybrid orbitals for the electronic geometry Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. orbital makes four, sp3 orbitals in a tetrahedral array. Molecular Orbital of Methane, CH4. 1. The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no . Ethene, sp2 hybridization with a pi bond. 1. 16.2.2017 · 1. Quick Review: Molecular Orbitals For A Simple Pi Bond. In the last post, we showed how to build a molecular orbital (MO) diagram for a typical C-C pi bond.. We saw that: The number of molecular orbitals equalled the number of contributing atomic orbitals. p-orbitals; 3p-orbitals; 3d-orbitals; 4f-orbitals; Compare shape and size of 1s, 2s and 2p orbitals; Molecular Orbitals. Hydrogen; Nitrogen; Fluorine; Ammonia; Methane; Ethylene (Ethene) Acetylene (Ethyne) Allene; Formaldehyde(Methanal) Acrolein; Carbon Monoxide; Hydrogen Fluoride; Allyl Anion; Butadiene; Benzene; Aromaticity of cyclic polyenes ...
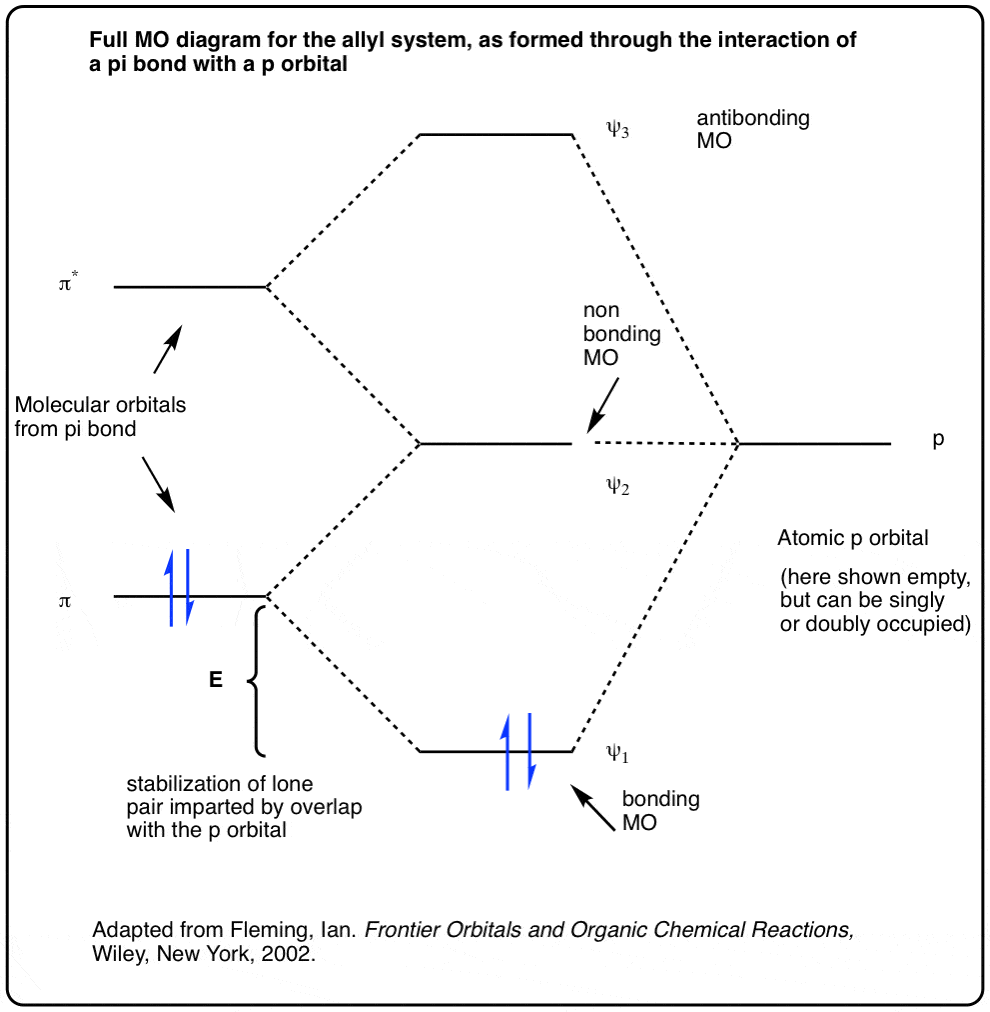
1 päivä sitten · Molecular Orbital diagram of CH4. The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen. ATOMIC ORBITALS of different atoms combine to create MOLECULAR ORBITALS. The number of ATOMIC ORBITALS = the number of MOLECULAR ORBITALS. Electrons in these . MOLECULAR ORBITALS . are shared by the molecule as whole . MOLECULAR ORBITALS. can be constructed from Linear Combination of Atomic Orbitals (LCAO) Molecular Orbital Theory. Y A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. 28.2.2017 · The Molecular Orbital Diagram of Butadiene, And How To Build It. In the last post in this series we built up the pi molecular orbitals of the allyl pi-system, consisting of three consecutive p orbitals in conjugation.In this article we will show how to build the pi molecular orbital diagram of butadiene.
It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. Generate the Molecular Orbitals for CH4(Td), CH4(D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us.
molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104! ! ! ! !Prof.
Likewise, the orbital correlation diagram for methane provides another example of the difference in electron density predicted by molecular orbital calculations from that of the localized bond model. Click on the compound names for these displays.
This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H (1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
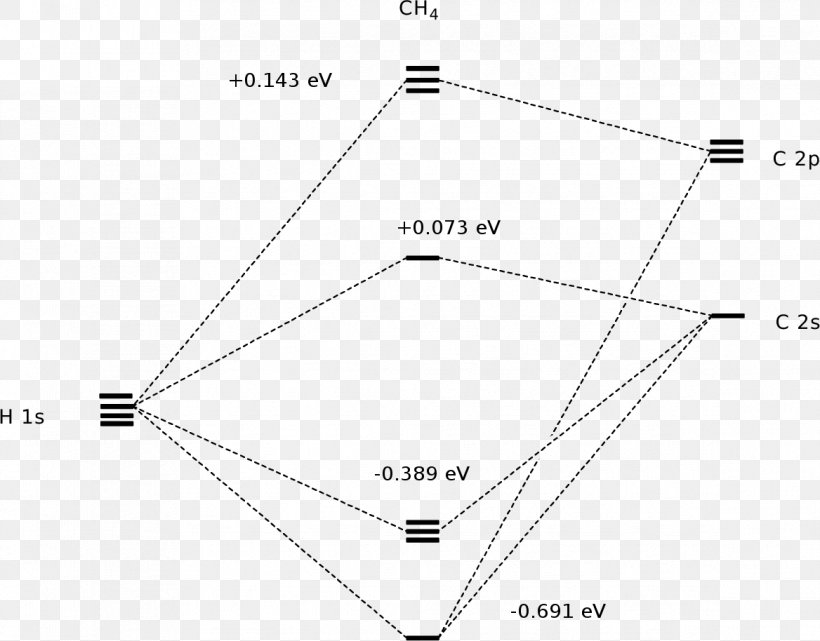
Methane has four valence molecular orbitals (bonding), consisting of one orbital with one nodal plane (lowest occupied) and three degenerate (equal energy) orbitals that do have a nodal plane. For the energy diagram and pictorial view of the orbitals - please see below:
Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic orbitals
Using the carbon and hydrogen atomic orbitals, methane, CH 4, is constructed by overlapping the carbon's one 2s and three 2p AOs with the four hydrogen 1s AOs. Methane's MOs have a topology similar to the AOs of carbon, but the structure can be very difficult to visualise, so the methane MO construction diagrams A, B and C (below) are shown with the AOs and MOs superimposed upon line structures of the methane.
Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
Molecular orbital (MO) theory uses a linear combination of atomic orbitals (LCAO) to represent molecular orbitals resulting from bonds between atoms. These are often divided into three types, bonding, antibonding, and non-bonding.A bonding orbital concentrates electron density in the region between a given pair of atoms, so that its electron density will tend to attract each of the …
• Example - In methane, CH 4, the sp3 hybrid orbitals point at the 1s hydrogen orbitals, and the atomic orbitals are added and subtracted to create molecular orbitals • The other resulting MO is higher in energy than the two atomic orbitals and is antibonding • Only the lower-energy orbital is populated with electrons in methane
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two
1 päivä sitten · C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. Here, bond strength depends on the overlapping degree which in turn depends on the spatial proximity of the combining atoms.
Methane Molecular Orbitals. In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered. A molecular orbital will be displayed by pressing the appropriate button.The different phases of the molecular orbitals are colored red and blue and are separated by nodal surfaces at which electron density is zero.
Molecular orbital theory is concerned with the combination of atomic orbitals to form new molecular orbitals. ... sp 3 An example of this is methane (CH 4). ... Write out the electron configuration diagram using molecular orbitals and calculate the bond order of HCl, NO-1, ...
This animation explains the methane molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the website:www.quimica3d.com
Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups.
This animation explains the methane molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the youtube channel Lili Tosta: htt...
Chapter 2. Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1: Classes of Hydrocarbons molecules that are made up of carbon and hydrogen
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
The three molecular orbitals (MOs) of methane in the ground electronic state (X(1)A(1)). namely, the core MO, 1a(1) and the valence MOs, i.e. 2a(1) and three-fold energy degenerate MOs 1t(2) are ...
The three molecular orbitals (MOs) of methane in the ground electronic state (X 1 A 1 ), namely, the core MO, 1 a1 and the valence MOs, i.e. 2 a1 and three-fold energy degenerate MOs 1 t2, are studied in both coordinate space and momentum space. They are compared with the corresponding atomic orbitals (AOs) of a 'free' carbon atom in its ground electronic state ( 3 P) using configuration averaged unrestricted HF (UHF) method.
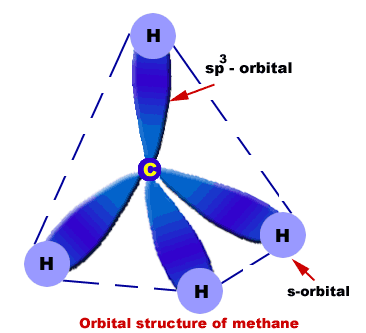
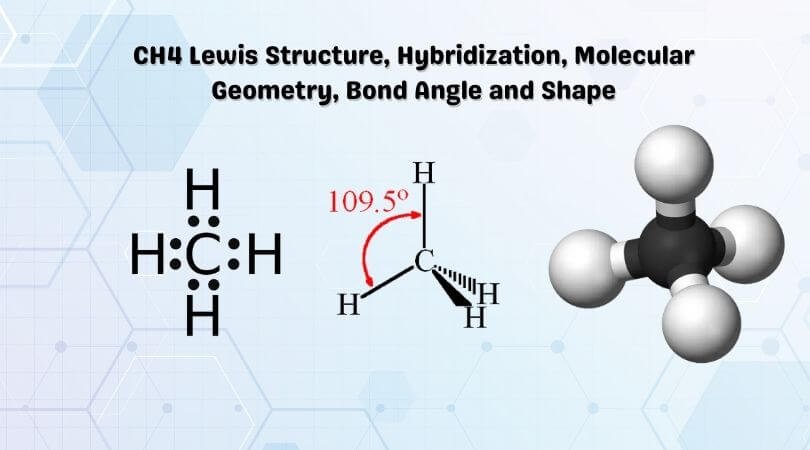
The resulting angle between orbitals is 109.5°. Four Hydrogen atoms bond with Carbon to give methane. Hydrogen's spherical 1s orbital merges with one of Carbon's sp 3 orbitals to form a new molecular bonding orbital with Hydrogen's nucleus embedded in it. The bond produced from the overlap of the two atomic orbitals is called a sigma bond (σ-bond).
• The carbon atom has one 2s orbital and three 2p orbitals • Each hydrogen atom has one 1s orbital • One can write the solution for the methane molecule as a linear combination of all available orbitals r c r r c s r c px r c py r c pz r j j s j 5 2 6 2 7 2 8 2 4 1
The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The two carbon atoms bond by merging their remaining sp 3 hybrid orbitals end-to-end to make a new molecular orbital. The bond formed by this end-to-end overlap is called a sigma bond. The bonds between the carbons and hydrogens are also sigma ...
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons.





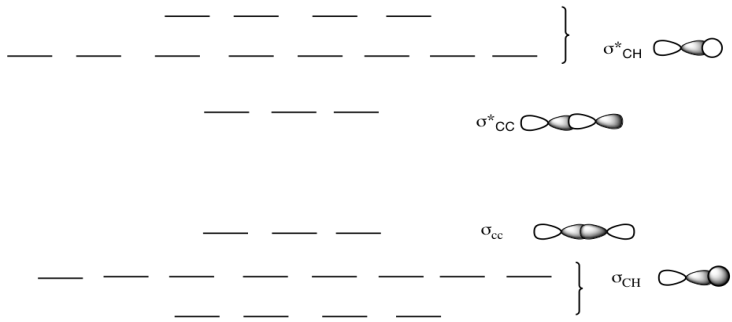









0 Response to "37 methane molecular orbital diagram"
Post a Comment