38 mo energy level diagram
Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
A molecular orbital energy level diagram just shows the energy levels in the molecule. Frequently, but not always, energy level diagrams are shown without any pictures of the orbitals, in order to focus attention on the energy levels, which in a fundamental way are the most important part of the picture.
Mo energy level diagram
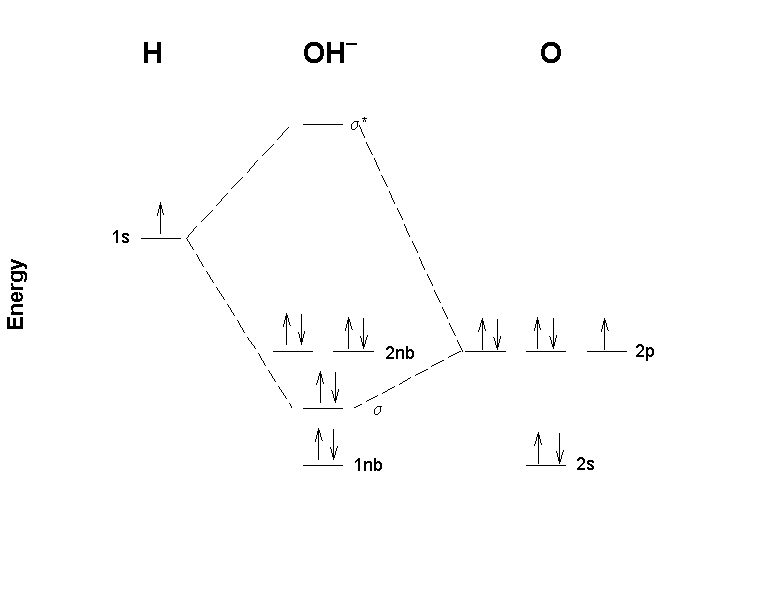
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine MO diagrams with energy values can be obtained mathematically using the Hartree–Fock method. The starting point for any MO diagram is a predefined molecular ...Basics · Diatomic MO diagrams · MO energies overview · Heteronuclear diatomics The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation, high resolution images and no watermark.
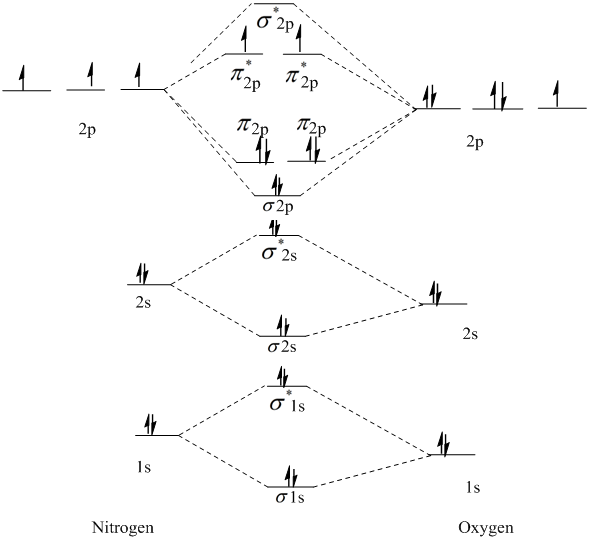
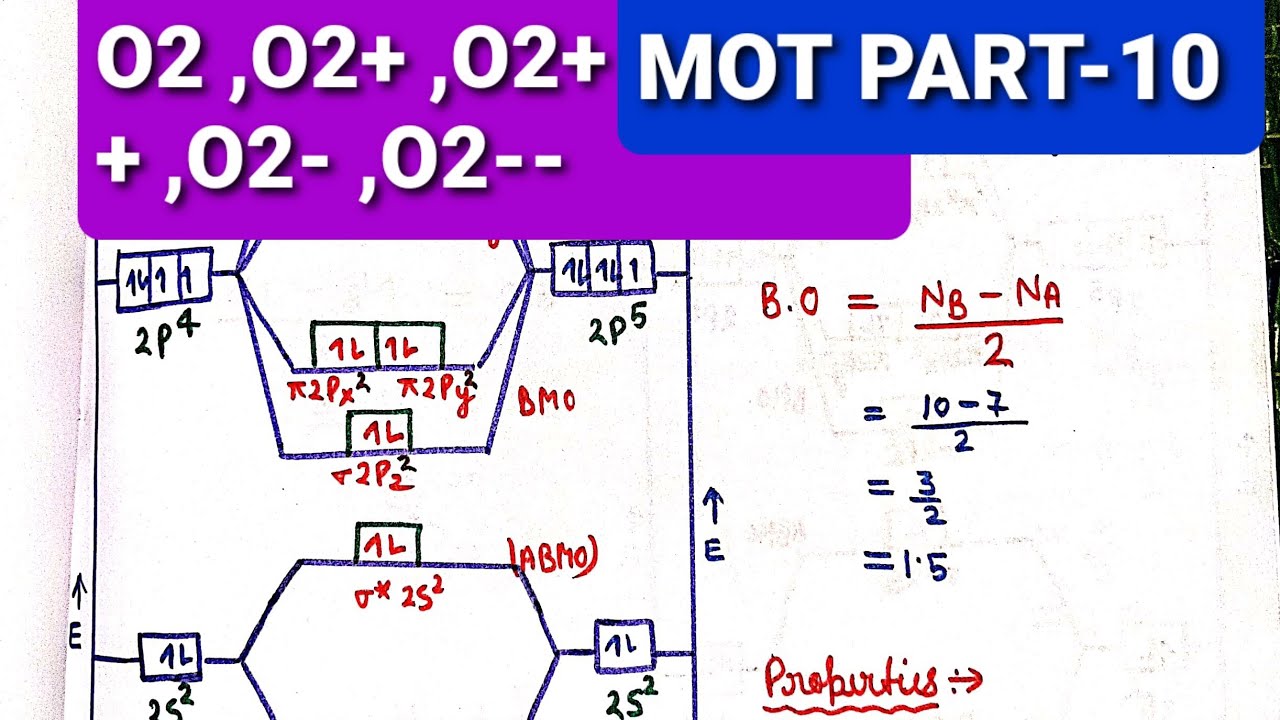
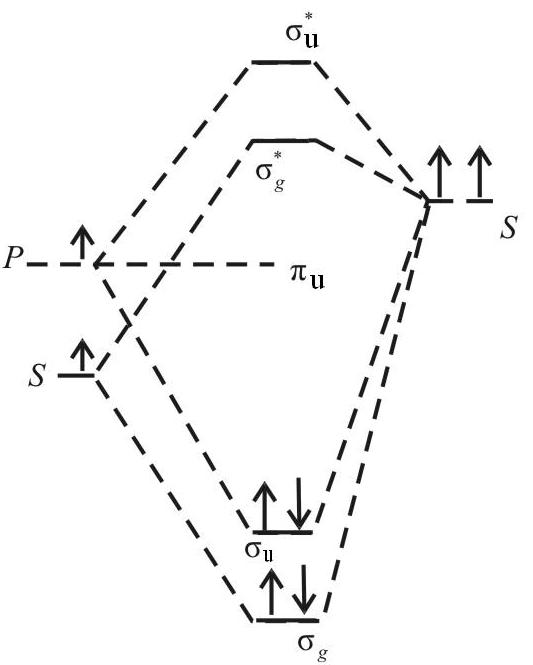
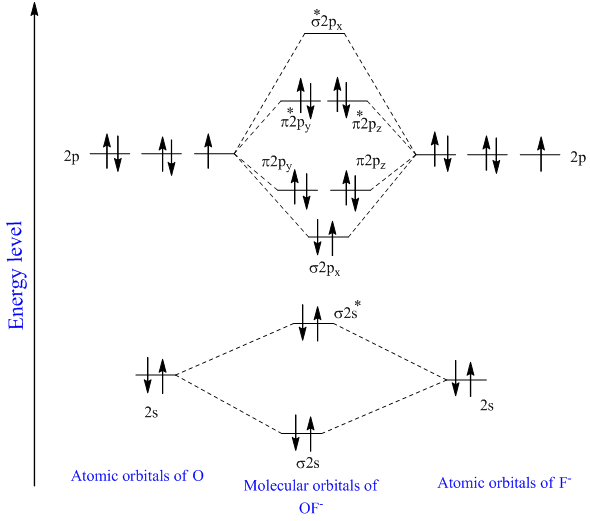
Mo energy level diagram. Energy level diagram for Molecular orbitals Last Updated on March 20, 2019 By Mrs Shilpi Nagpal 18 Comments Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure 13 ... Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. σ bonding MO that is lower in energy than the constituent 1s AOs and an antibonding σ* MO that is at a higher energy than the 1s AOs. [1] Each. The molecular orbital energy level diagrams for H2, H2. +, H2. – and O2 are shown below. The MO diagram is complete when all of the valence electrons are used. Let's demonstrate these principles with a couple problems. #1. Draw the MO diagram for `B_2`. First step is to determine which MO diagram we're using. In this case, we're using the standard one. Draw out the MO diagram and label in the valence electrons.
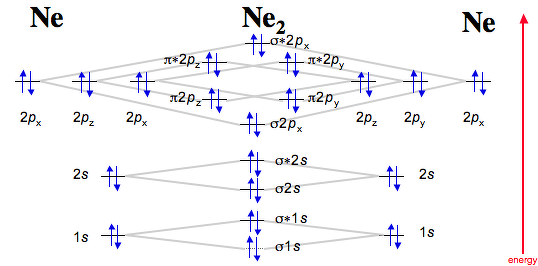
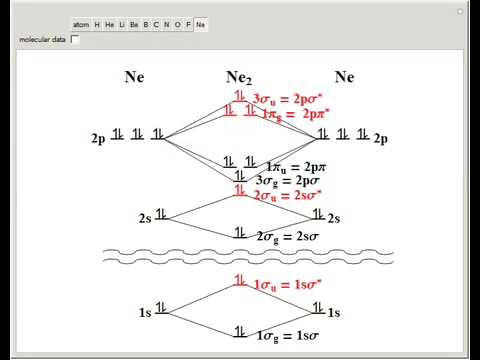
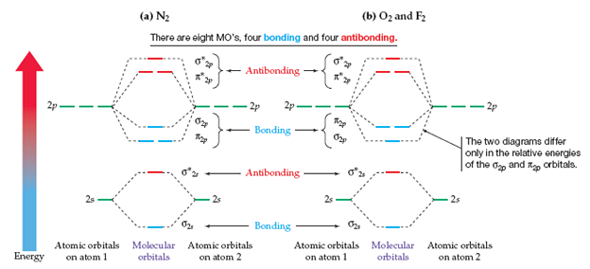
M.O.Energy Level Diagram for A. 2 (A = Li, Be) Li. 2. Only two valence electrons, i.e. σ. s 2. σ * s 0. Bond order =1. Li. 2 . exists in gas phase over metallic lithium. Diamagnetic "Be. 2 " σs. 2. σ * s. 2 . Bond order = 0 - no net bonding energy, so molecule does not exist. Beryllium in gas phase is monatomic. Use Aufbau, Pauli, Hund ... There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation, high resolution images and no watermark. MO diagrams with energy values can be obtained mathematically using the Hartree–Fock method. The starting point for any MO diagram is a predefined molecular ...Basics · Diatomic MO diagrams · MO energies overview · Heteronuclear diatomics
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine




















![DFT energy level diagram for [Mo 5 NbI 8 (CN) 6 ] 3 ...](https://www.researchgate.net/profile/Sofya_Artemkina/publication/257859841/figure/download/fig4/AS:297638919458823@1447974056584/DFT-energy-level-diagram-for-Mo-5-NbI-8-CN-6-3-schematic.png)



0 Response to "38 mo energy level diagram"
Post a Comment