37 lewis diagram for ch4
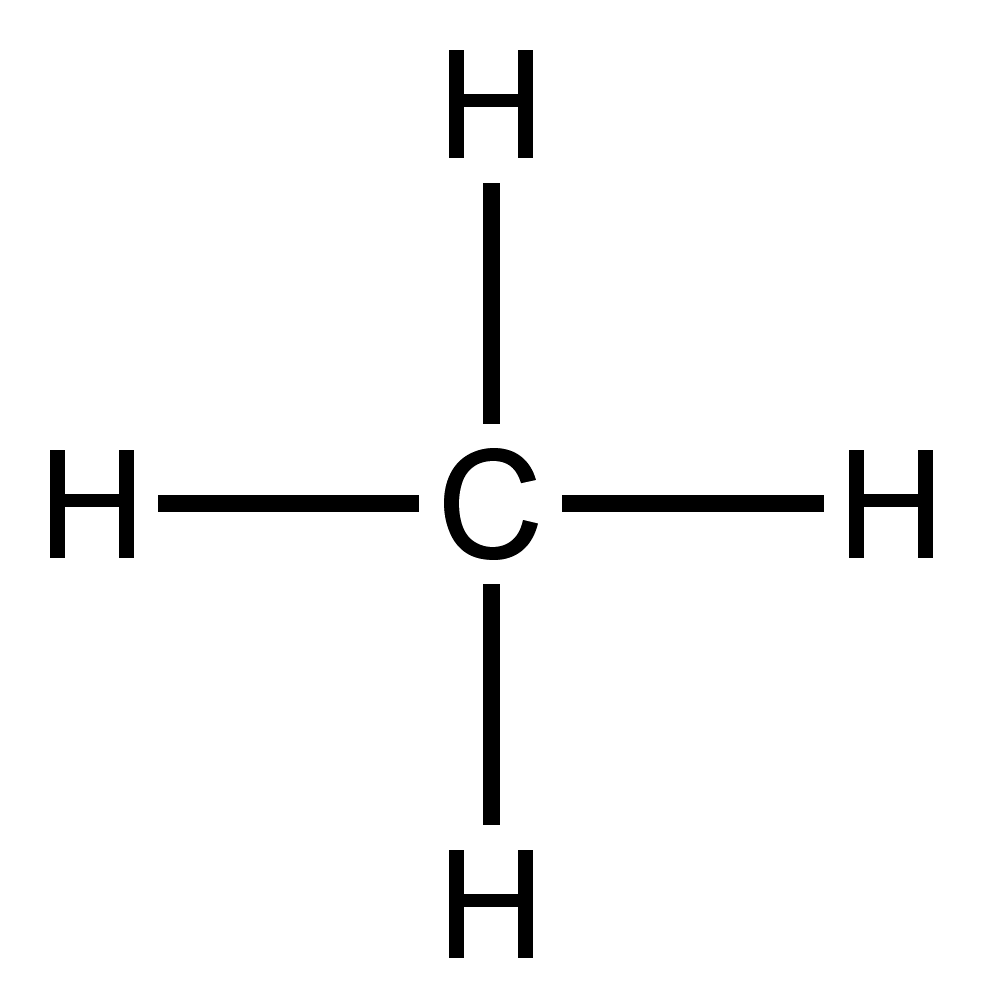
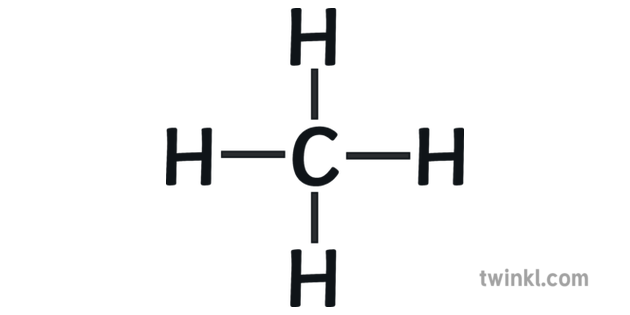
CH4 lewis structure. As you see in this CH4 lewis structure, carbon central atom and hydrogen atom completed their octet, and everything looks fine, but, for the sake of satisfaction, we should also determine the formal charge in the above structure to know whether it is stable or not. A single Lewis structure can be used to represent the bonding in CH4.There are 2 equivalent Lewis structures for nitryl chloride where the double bond to oxygen can be placed on either of the ...
Lewis dot diagram for hcn. The NaOH molecule will aid in the demonstration of intermolecular forces that can act on a jadeite molecule. This is because carbon has four valence electrons forming four bonds and in a three-dimensional space, a tetrahedral shape allows for the bonded electrons to be furthest away from each other.

Lewis diagram for ch4
The Lewis diagram is also drawn by presenting electrons in the form of points forecasting the creation of bonds. These lines also specify which type of bond has been generated to assist in creating core atoms hybridization. LEWIS STRUCTURE OF METHANE (CH4) H.. H: C: H.. H. This is the Lewis Dot representation of methane. Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite. Transcribed image text: To answer the questions, interpret the following Lewis diagram for CH4 H-C-H HICOH For the central carbon atom: The number of lone pairs The number of single bonds = The number of double bonds EE Submit Answer Retry Entire Group 2 more group attempts remaining To answer the questions, interpret the following Lewis diagram for C2H4 H H H H The number of lone pairs The ...
Lewis diagram for ch4. Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a … Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. 10+ Lewis Dot Structure For Ch4. Let's go ahead and look at another example. Drawing c is a lewis electron dot structure for methane. The condensed formula for ethane makes it easy to forget that there is a bond between the two carbons. Lewis structure is basically a graphic representation of the electron distribution around an atom. Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. The Lewis Dot Structure for CH4 is shown above.
Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. The largest natural reservoir of CH4 lies in the seafloor. This underwater CH4 reservoir is called methane clathrate (methane ice), and it is trapped in an ice-like crystal structure. Man-made CH4 mainly comes from the oil and gas industry. CH4 is a greenhouse gas, and scientists have detected its presence on the planet Mars. Molecular orbit diagram of CH4 The molecular orbit diagram helps with determining exactly how mixing and overlapping have taken location in a molecule come conclude upon the hybridization type. As every the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with 4 1s atom orbitals of the hydrogen. Ch4.5 Lewis Symbols and Lewis Structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 15 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 15.
Acetic acid (CH3COOH) lewis structure. So, the above lewis structure of Acetic acid is the best and stable as all atoms have formal charge zero. I really hope you enjoyed the procedure of making a lewis diagram with all concepts and possible explanations. ⇒ Lewis structure of Acetate ion(CH3COO-) – CH4 Lewis Structure & Molecular Geometry. Methane ( CH4) is a colourless, odourless, and highly combustible gas that is utilized to generate energy and heat houses all over the world. CH4 Lewis structure comprises two different atoms: Carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5° degrees. Lewis structure of ch4. The compound is one of the. A covalent molecule contains at least two atoms sharing some number of valence electron pairs through one or more covalent bonds. The physical properties of the molecule like boiling point surface tension etc. Remember that uncharged carbon has 4 bonds and no lone pairs and hydrogen has one ... Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Cl2 Br2 H2 O2 N2 HCl DOUBLE bond atoms that share two e- pairs (4 e-) O O TRIPLE bond atoms that share three e- pairs (6 e-) N N Draw Lewis Dot Structures You may represent valence electrons from different atoms with the following symbols x, , CO2 NH3 Draw the Lewis Dot Diagram for polyatomic ions Count all valence e- needed for covalent bonding Add or subtract …
CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
26.12.2021 · MO Diagram of CCl4. A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4’s and CH4 MO diagram looks like.
Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen). For the O2 Lewis structure, calculate the total number of valence electrons for the ...
Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .
Lewis didn’t simply figure out how to diagram molecules with dots, he was also the one who explained the covalent bond, coined the term “photon,” figured out how electrons pair together, and, among other things, he inspired Linus Pauling (1901–1994), another extraordinary chemist, to study the nature of chemical bonding.
Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure There are following specifications in the lewis structure of methane. Four hydrogen atoms have made bonds with center carbon atom and all those bonds are single bonds. No lone pairs on valence shells of carbon and hydrogen atoms.
What is the correct Lewis dot diagram for CH4? Next Worksheet. Print Lewis Structures: Single, Double & Triple Bonds Worksheet 1. How do you represent a triple bond in a structural ...
Answer (1 of 2): I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydro...
The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ...
In this example, we can draw two Lewis structures that are energetically equivalent to each other — that is, they have the same types of bonds, and the same types of formal charges on all of the structures.Both structures (2 and 3) must be used to represent the molecule's structure.The actual molecule is an average of structures 2 and 3, which are called resonance structures.
The Lewis diagram for CH4 is: H-C-H The electron-pair geometry around the C atom in CH4 is There are lone pair(s) around; Question: Please note that "geometry" refers to the molecular or ionic geometry. A. The Lewis diagram for SC12 is: The electron-pair geometry around the Satom in SCl2 is | There are lone pair(s) around the central atom, so ...
Dec 28, 2021 · CH4 Lewis Structure, Molecular Geometry, and Hybridization. Methane or CH4 is a naturally occurring gas and relatively abundant on the Earth, making it an economically efficient fuel. As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production. It is one reason why overproduction ...
Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the.
For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ]. Explanation:
Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the . I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane.
In the given molecule methane, CH₄: the valence configuration for C = 2s²2p² the valence configuration for H = 1s¹ Since two electrons are required to form a bond, the C atom in methane can form one bond with each of the 4 H atoms. Therefore, in the Lewis diagram, the C atom will be in the center surrounded by the 4 H atoms. Advertisement Survey
Check me out: http://www.chemistnate.com
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Transcribed image text: To answer the questions, interpret the following Lewis diagram for CH4 H-C-H HICOH For the central carbon atom: The number of lone pairs The number of single bonds = The number of double bonds EE Submit Answer Retry Entire Group 2 more group attempts remaining To answer the questions, interpret the following Lewis diagram for C2H4 H H H H The number of lone pairs The ...
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite.
The Lewis diagram is also drawn by presenting electrons in the form of points forecasting the creation of bonds. These lines also specify which type of bond has been generated to assist in creating core atoms hybridization. LEWIS STRUCTURE OF METHANE (CH4) H.. H: C: H.. H. This is the Lewis Dot representation of methane.




























0 Response to "37 lewis diagram for ch4"
Post a Comment