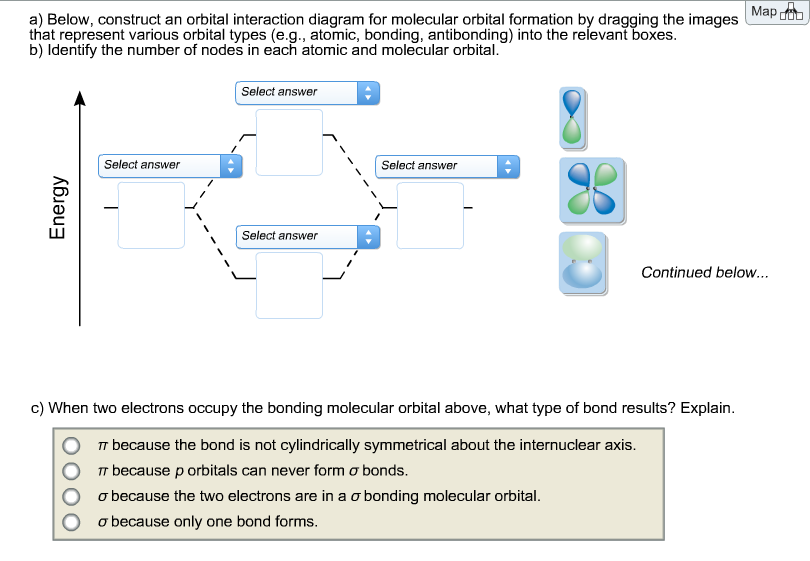
41 below construct an orbital interaction diagram
When I first heard about ATCOR, I didn't believe it. I figured it was a wildly unrealistic principle, and I couldn't find many resources about it to understand it. I thought this itself was very suspicious, since it was so "overpowered" if true, I would have expected people to have prepared detailed resources long ago to shout from the rooftops. However, I think I've finally just about managed to wrap my head around it for the most part, and now I feel like it could be surprisingly robust. I ...
arXiv:2112.01677v1 [cond-mat.str-el] 3 Dec 2021 Microscopic Theory of Superconducting Phase Diagram in Infinite-Layer Nickelates T. Y. Xie1 ,∗, Z. Liu2, Chao Cao3, Z. F. Wang 2, J. L. Yang , W. Zhu4 1 Zhejiang University, Hangzhou, 310027, China 2Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui …
Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim...

Below construct an orbital interaction diagram
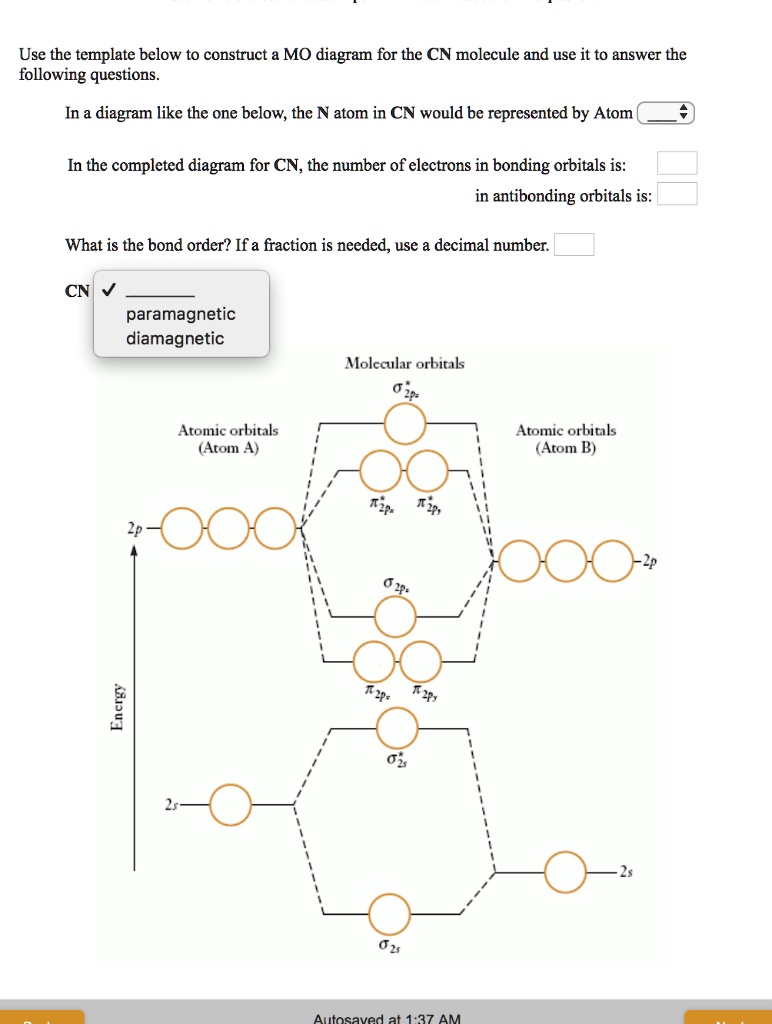
04.03.2021 · Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those …
01.12.2021 · The optimized liquid interaction parameters are listed in Table 3. Fig. 12 shows the phase diagram calculated using all interaction parameters obtained for all the phases considered in the Sm–Ti system. At the temperature below 1000 K, phase separation between rhombohedral-Sm and hcp-Ti is predicted.
The energy of an electron is mainly determined by the values of the principal and orbital quantum numbers. The principal quantum number is simply expressed by giving that number, but the orbital quantum number is denoted by a letter. These letters, which are derived from the early days of spectroscopy, are s, p, d and f, which signify that the orbital quantum numbers l are 0, …
Below construct an orbital interaction diagram.
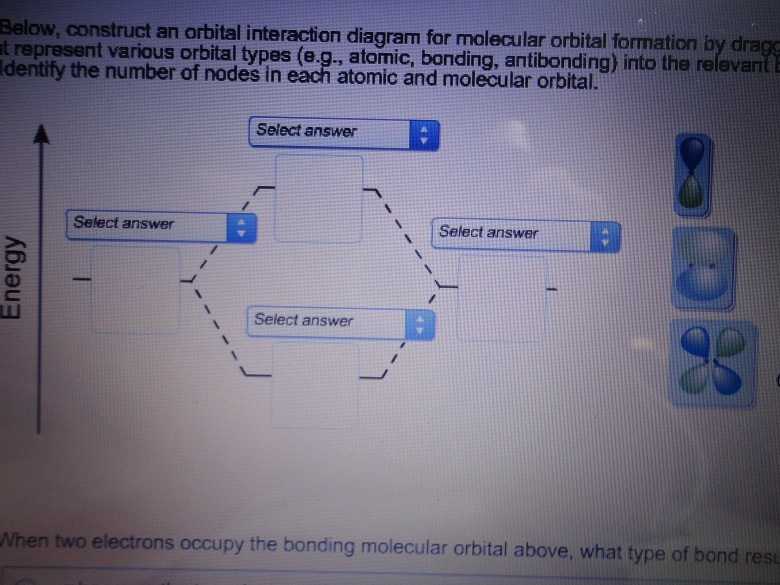
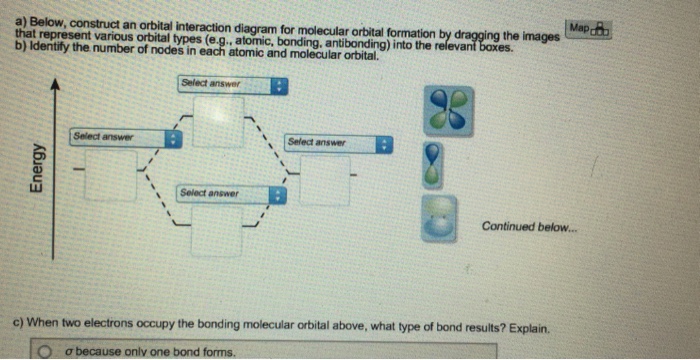
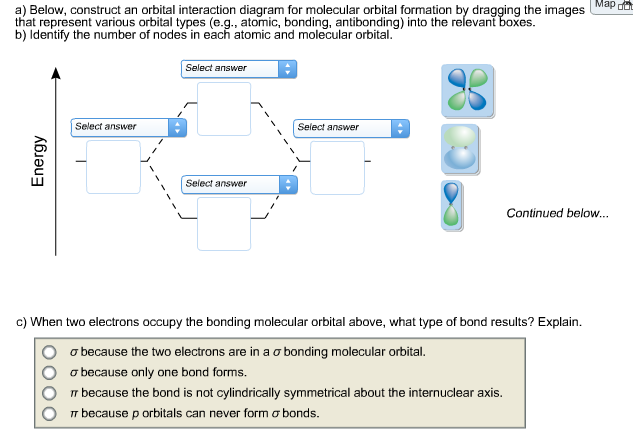
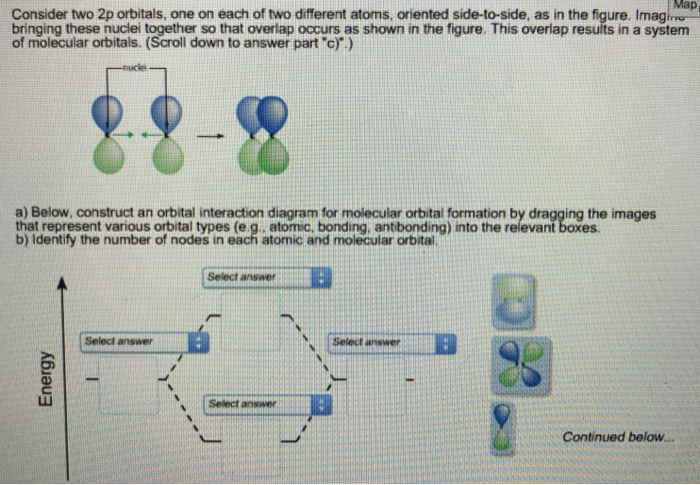
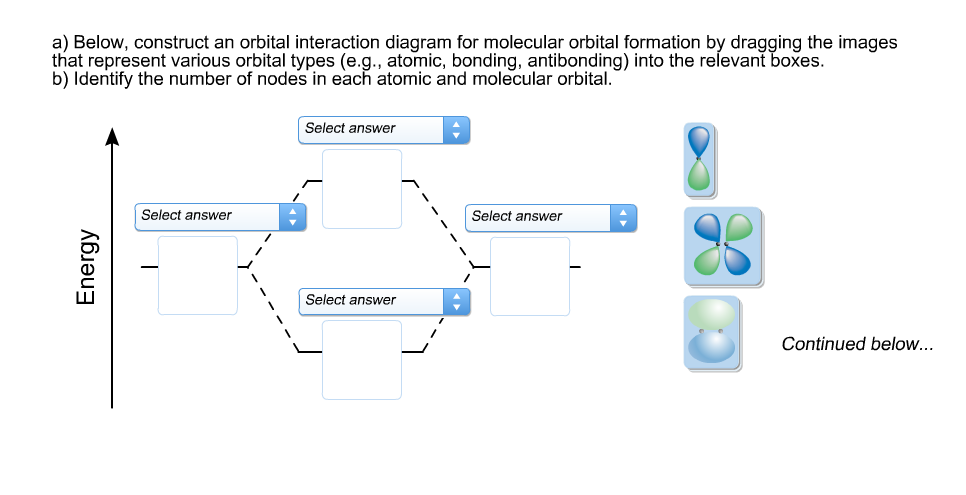
Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital. When two electrons occupy the bonding molecular orbital above, what type of bond results?
Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim...
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
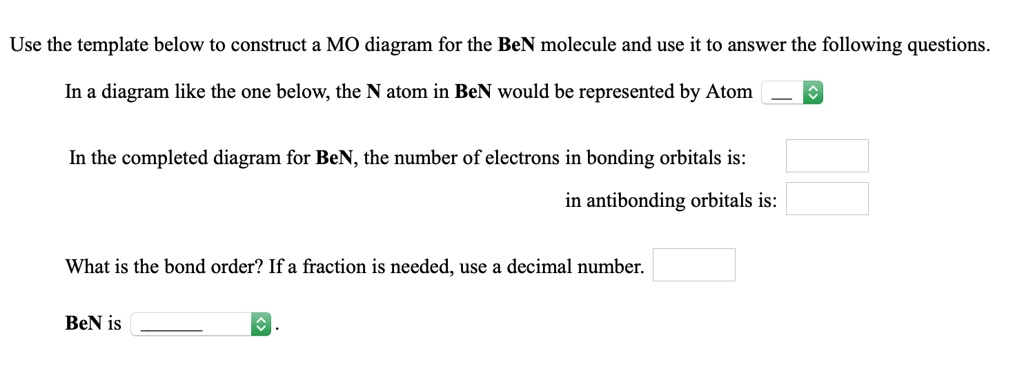
21.11.2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory.
[First](https://www.reddit.com/r/HFY/comments/ib293n/the_shoulders_of_orion_ch_1_first_contact/) [Previous](https://www.reddit.com/r/HFY/comments/m9fg9g/the_shoulders_of_orion_ch_6_claims/) Councilor Halon Va, former High Admiral of the combined Federation fleets, sat silently, observing the spectacle before him as his shuttle cruised towards the human ships. When news had first broken of the attack on Chelsith, human relief efforts had materialized at the edge of the system before even ...
It is possible for the 2s orbital on one atom to interact with the 2p z orbital on the other. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
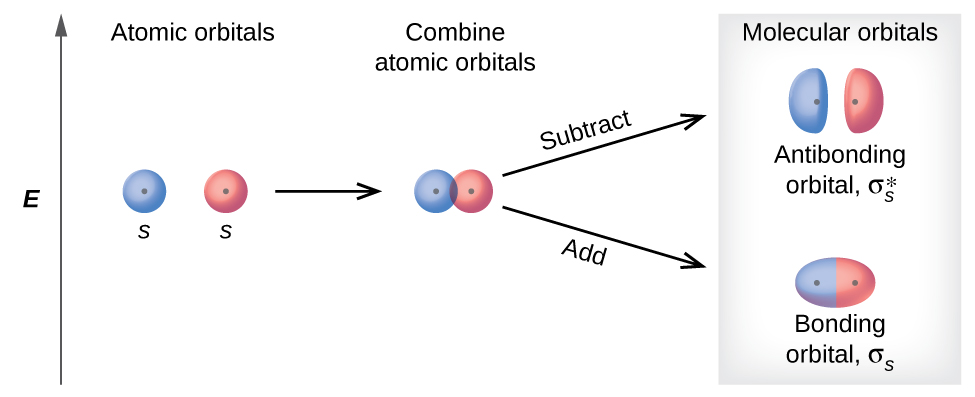
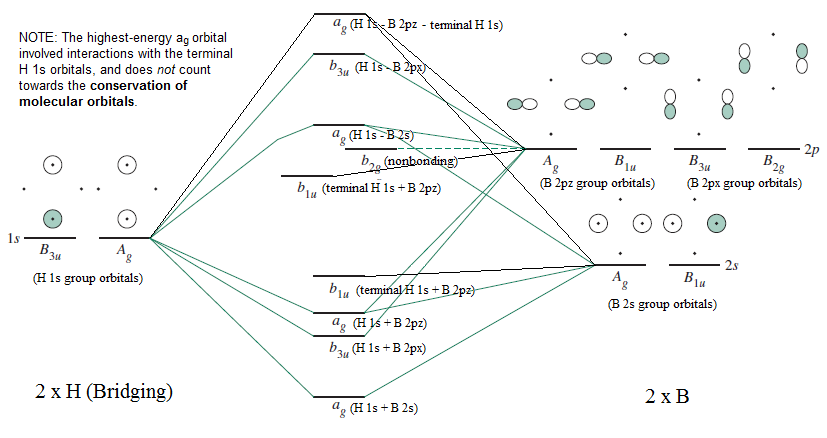
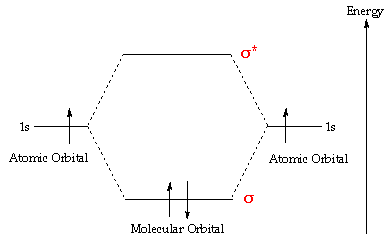
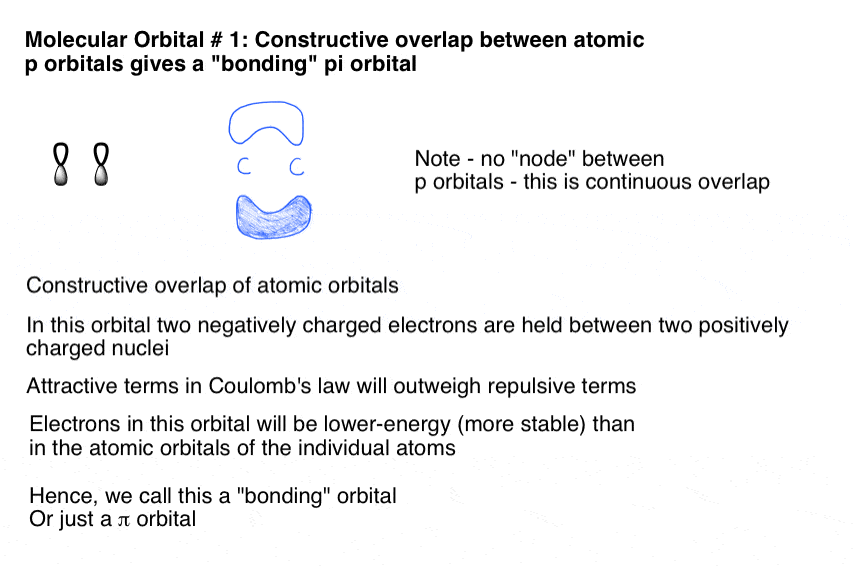
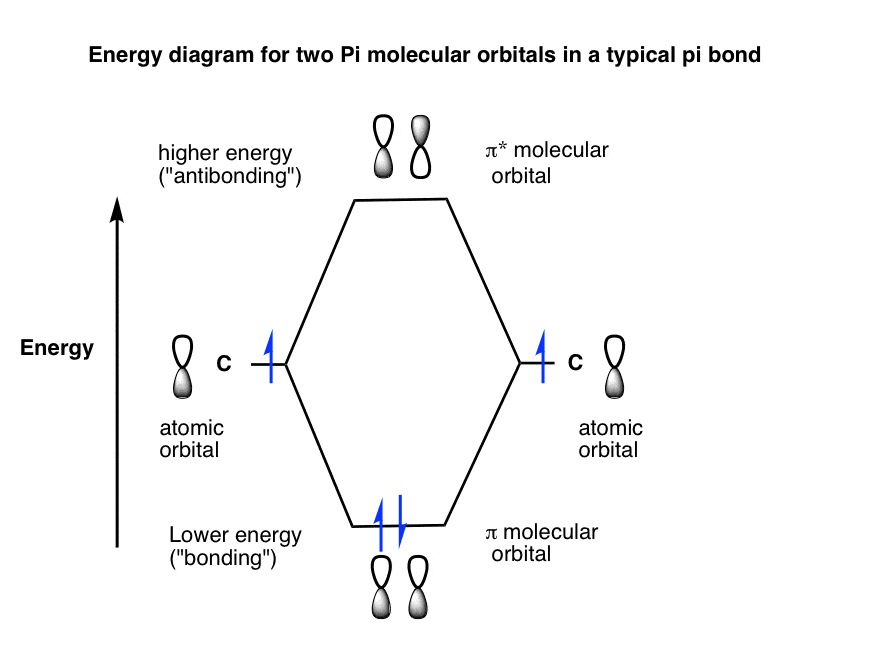
A molecular orbital interaction diagram shows how atomic or molecular orbitals combine together to make new orbitals. Sometimes, we may be interested in only the molecular orbital energy levels themselves, and not where they came from. A molecular orbital energy level diagram just shows the energy levels in the molecule. Frequently, but not always, energy level …
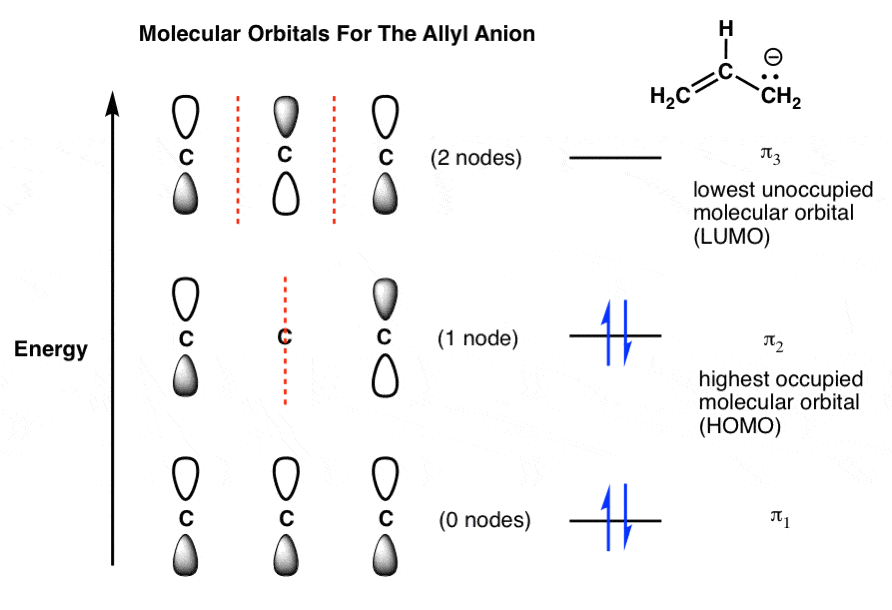
As shown in the diagram below, the contribution to the electron density at a specific atom from a single electron in the BMO is given by the square of the coefficient of that atom in the BMO. For atoms 1 and 3, that is 1/4; for atom 2 it is 1/2. Since there are two electrons in . this MO, we must double this, giving 1/2 for C1 and C3 and 1.0 for C2. To be neutral, each carbon should have ...
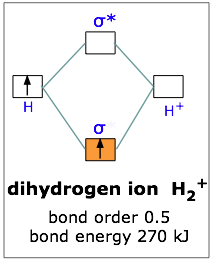
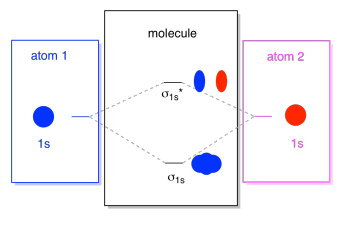
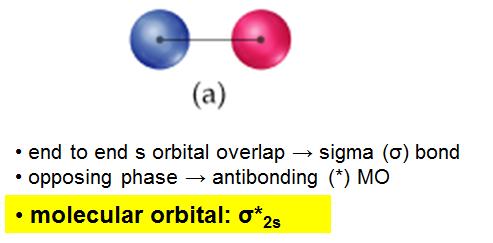
The interaction of the two bonded atoms with the bonding electrons produces a more stable arrangement for the atoms than when separated. Electrons usually occupy these orbitals. A sigma bonds is always the first bond formed between two atoms. Sigma star (σ*) antibonding molecular orbital – Normally this orbital is empty, but if it should
Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim...
orbital residing in the unit cell at lattice site R. Since there is only one Bloch basis orbital for this problem, the secular determinant for this problem is trivial, but we still must evaluate H(k): H(k) = e2 ik R R HR; where H = (0)H (R) The diagram here shows the set of coefficients for the factor e2 ik R in this equation (the components of ...
Orbital Interaction Diagram 1. Plot atomic valence orbital en ergies (or fragment orbitals for More complex molecules). 2. Determine which orbitals can interact (those with S 0). 3. Determine magnitude of each interaction: scales directly with magnitude of overlap scales inversely with orbital energy difference 4. Plot MO energies and draw orbitals
The key to suspense is that it sets up a question, or several that the audience hopes to get an answer to, and delays that answer while maintaining their interest and keeping them guessing. The boy gives birth to demons, he can't help himself. That boy gets chased until he no longer runs, no longer walks, no longer stands, no longer thinks, and holds his breath for a few seconds. That boy sacrifices himself so that paradoxically the man can save the boy. Life rewards practical self-justifying o...
(Scroll down to answer part "c)".) Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Dashed lines show which of the atomic orbitals combine to form the molecular orbitals. For each pair of atomic orbitals that combine, one lower-energy (bonding) molecular orbital and one higher-energy (antibonding) orbital result. Thus we can see that combining the six 2p atomic orbitals results in three bonding orbitals (one σ and two π) and three antibonding orbitals (one σ* and two π*).
Dec 11, 2019 · Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.















0 Response to "41 below construct an orbital interaction diagram"
Post a Comment