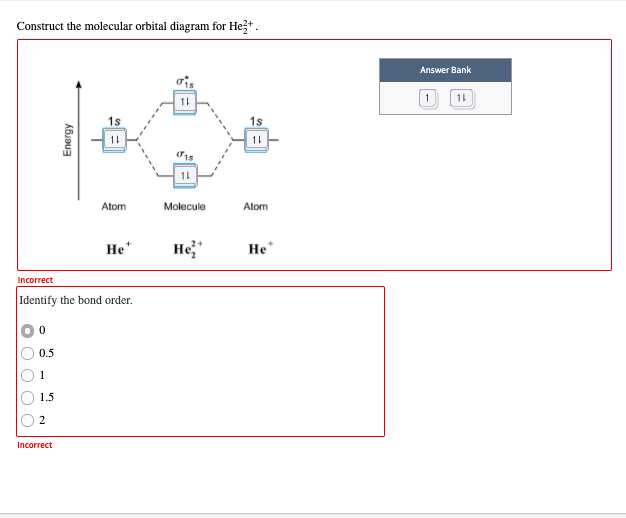
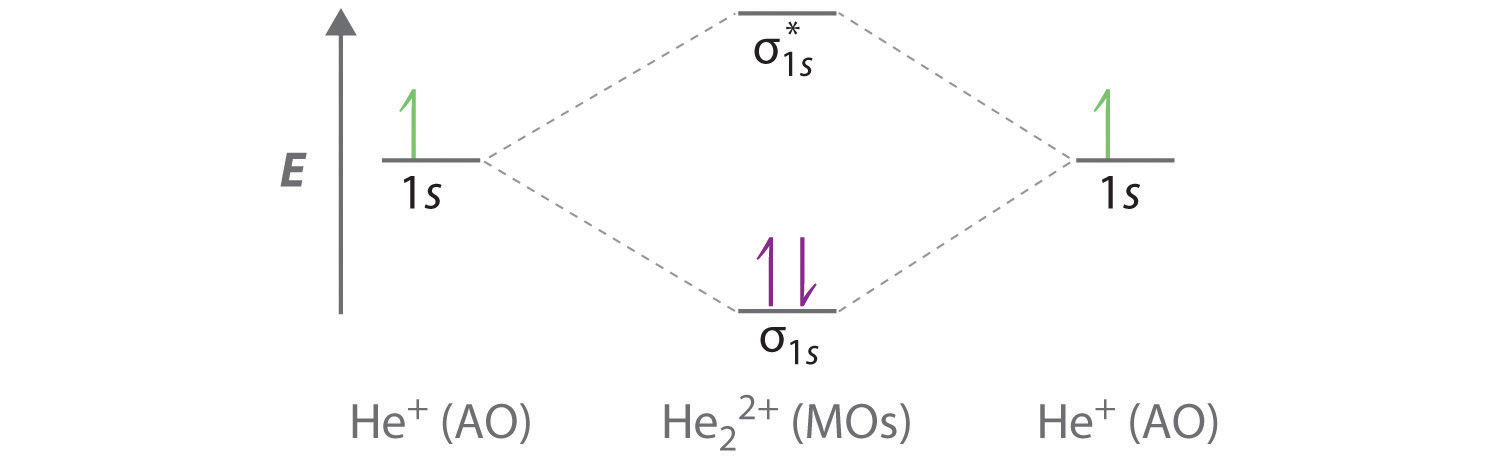
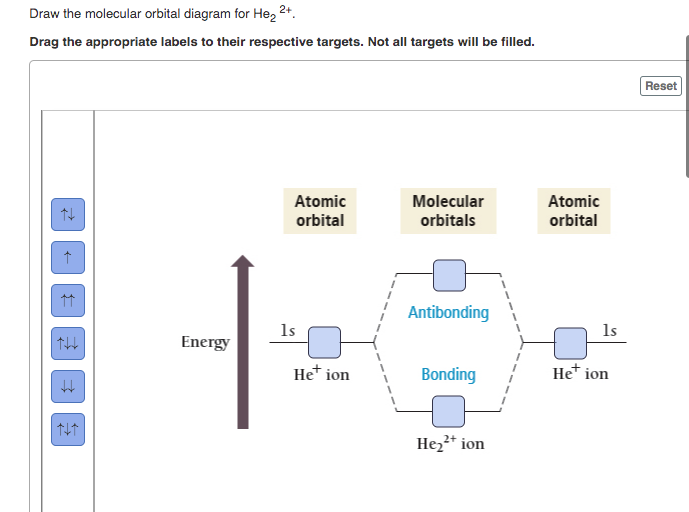
39 molecular orbital diagram for he2 2+
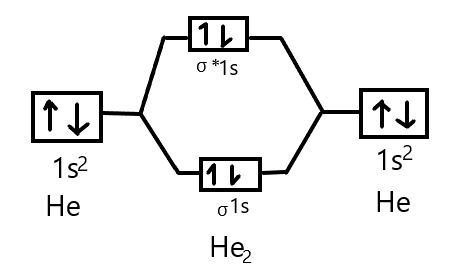
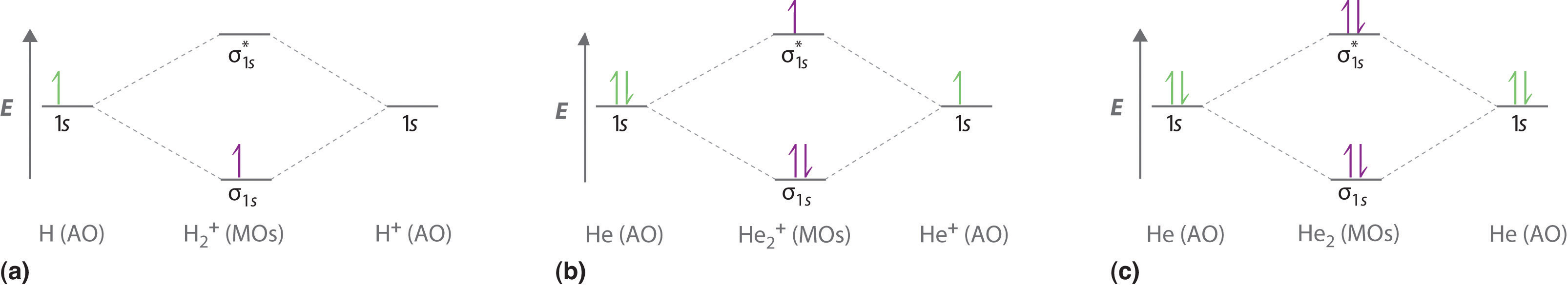
The energy level diagram for he2 is shown above the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecu...
Molecular Orbital Diagram For He2 2. Image by Wiringall.com. Iit Jee Bonding In Homonuclear Diatomic Molecules Li2 Li2 Be2 B2.
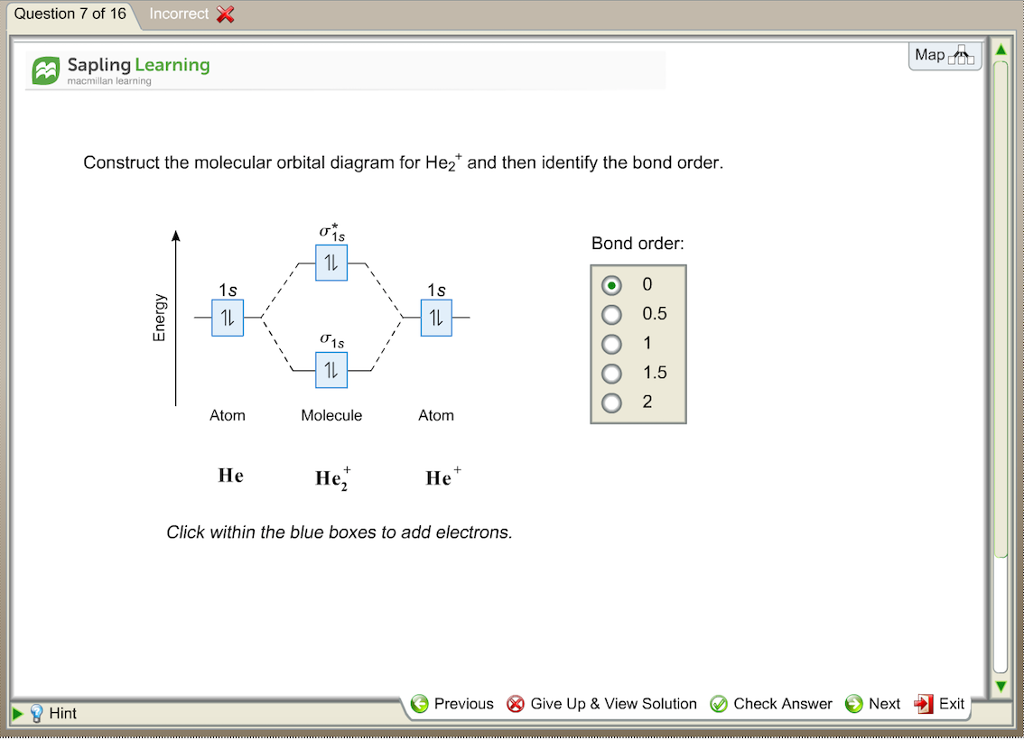
Draw a molecular orbital diagram for He2. Use this diagram and bond order calculations to explain if He2 is more or less stable than the ions He2+ and He2−. I have done He2 and He2+ and the bond order calculations for both. I just wanted to know why you cant do the same for He2-
Molecular orbital diagram for he2 2+
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the #1s# orbitals, because they do not contain the valence electrons.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Similarly, the molecular orbital diagrams for homonuclear diatomic compounds of the alkaline earth metals (such as Be 2 ), in which each metal atom has an ns 2 valence electron configuration, resemble the diagram for the He 2 molecule in part (c) in Figure [Math Processing Error].
Molecular orbital diagram for he2 2+.
Molecular Orbital Diagrams (H2 and He2) One of the strengths of molecular orbital theory is its ability to describe the energy of both occupied and unoccupied molecular orbitals for The molecular orbital diagram for the second row homonuclear. Chapter 9. Theories of Chemical Bonding.
Ha molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular...
Answer : According to the molecular orbital theory, the general molecular orbital configuration will be, As there are 7 electrons present in nitrogen. The bond order of is, 3. The molecular orbital diagram of are shown below. laminiaduo7 and 94 more users found this answer helpful.
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short Dihelium (He-He) is a hypothetical molecule and MO theory helps to explain why dihelium does not N2 Molecular Orbital Diagram. With nitrogen, we see the two molecular orbitals mixing and the...
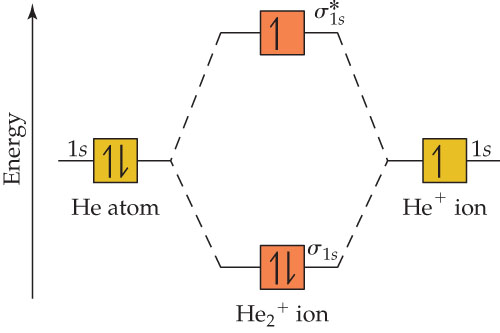
Molecular orbital diagram has been drawn for the given molecule . This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each.
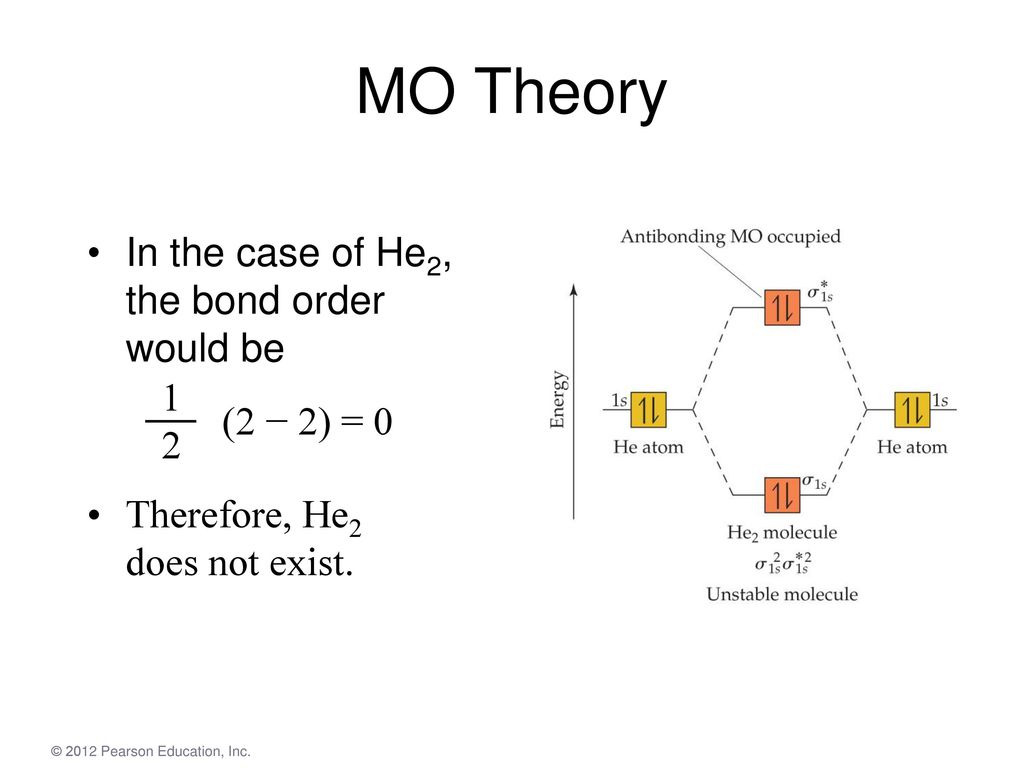
In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this The third diagram hypothesizes the molecule dihelium (He2). A bond order of zero is obtained by placing the available electrons in the bonding and...
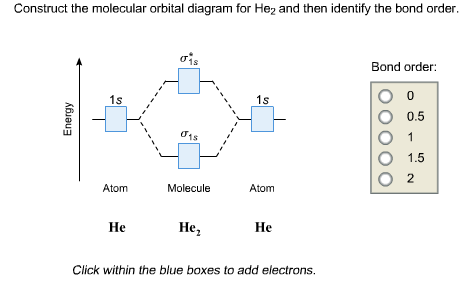
Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Molecular orbital confuguration of Ne2 is. In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. The binding is just very weak, and I think there are almost no He2 molecules till even lower temperatures than for neon.
Figure 8.36 The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding He2 is not possible. Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at The molecular orbital energy-level diagram, which is a diagram that shows the relative...
0:15 Molecular Orbital Diagram of Hydrogen Molecule 1:39 Molecular Orbital Diagram of Helium Molecule 2:54 Molecular Orbital ... This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule. The bond order of He2 is calculated ...
- MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
How to write simple Molecular Orbital Diagrams and determine the Bond order.
Nonbonding sigma is occupied and then the sigma orbital is occupied. C would this molecule exist. Do He2 He2 He2 2 Exist St...
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Molecular orbitals of H2 and He2. The procedure can be introduced by considering the H2 molecule. Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and antibonding (2σ) molecular...
He2 Molecular Orbital Diagram Free Wiring Diagram. Chapter 9 Molecular Geometry And Covalent Bonding Models. Chapter 6 Molecular Structure. Molecular Orbital Theory Grandinetti Group. Constructing The Hf Molecular Orbital Energy Level Diagram.
Simple molecular orbital diagrams. Dihydrogen and its ion H2+. Dihelium He2. As two H nuclei move toward each other, the 1s atomic orbitals of the isolated atoms gradually merge into a new molecular orbital in which the greatest electron density falls between the two nuclei.
Understanding FHF- molecular orbital diagram? Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion. Hot Network Questions.
This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
Molecular Orbital Diagram For He2 - Wiring Site Resource. 3 hours ago Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons...
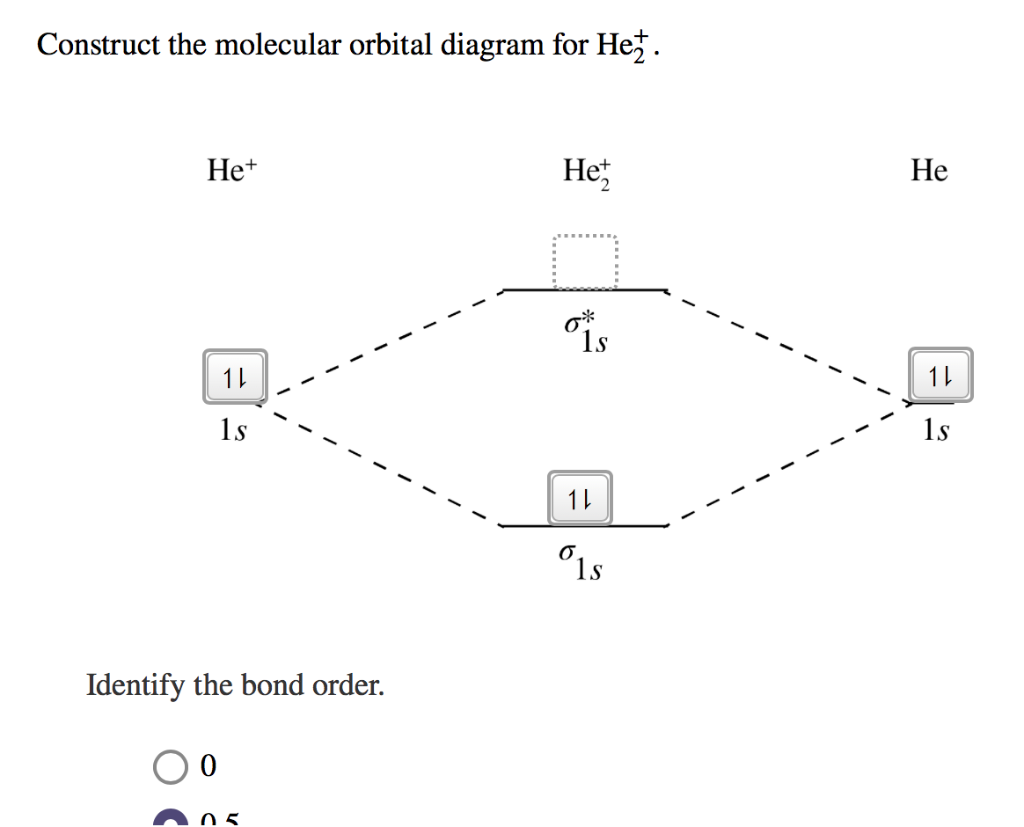
Transcribed image text : Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons.
He2 2 Molecular Orbital Diagram. Written By JupiterZ Monday, November 9, 2020 Add... Https Thinfilmsliterature Files Wordpress Com 2017 11 10 5 Molecular Orbital Theory Pdf Energy Level Diagram For Molecular Orbitals Chemical Bonding And
2 he2 has bond order 0 2 22 0 and we can make h. The molecular orbital approach is one explanation for the ceh h bond.
In a bonding molecular orbital, the electron density is high between the two atoms, where it stabilizes the arrangement by exerting a strong attraction for both nuclei. The energy level diagram for He2 is similar to that for H2 except that it has two more electrons.





















0 Response to "39 molecular orbital diagram for he2 2+"
Post a Comment