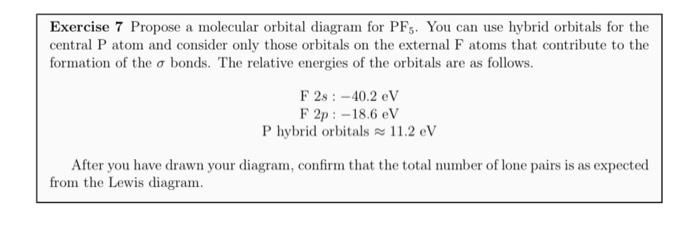
37 pf5 molecular orbital diagram
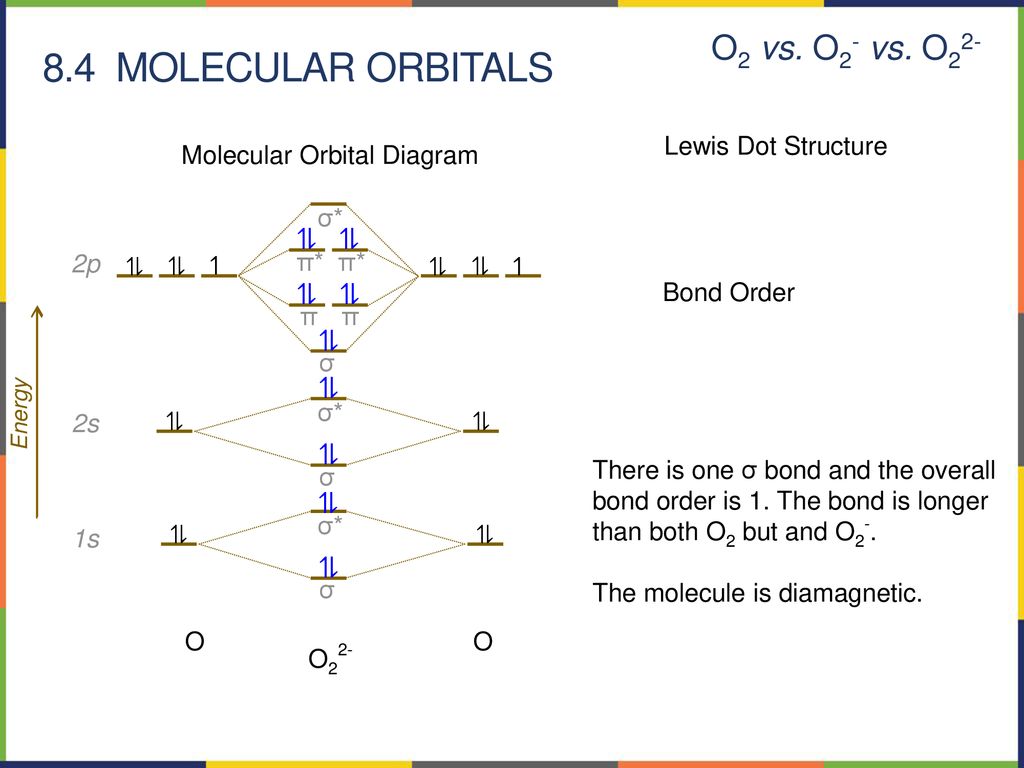
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon. PF5, and SF6. Guidelines for applying the VSEPR Model to predict molecular geometry. 1. Determine the Lewis structure. 2. Determine the central atom and sum ...
May 11, 2012 — An MO energy level diagram for a AX ... Molecular orbitals for σ bonding in PF5 ... An approximate MO diagram for a tetrahedral AX.75 pages
Pf5 molecular orbital diagram
PF5 SF6 elements from the third period on have unfilled d orbitals that can acomodate additional electrons. Case they do not exceed the octet rule. With this model we can draw a series of resonance structures as shown below for PF5. A On your work draw the Lewis structure of phosphorous pentafluoride and label the formal charge of any atom with ... Nov 5, 2013 — bond (VB) theory and molecular orbital (MO) theory. ... This approach has been used successfully to describe the bonding in PF5 (Scheme.4 pages Academia.edu is a platform for academics to share research papers.
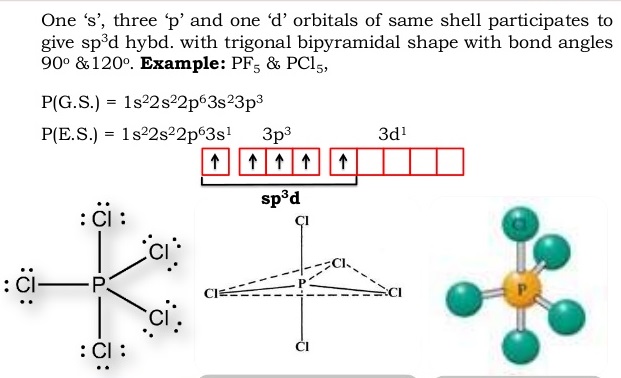
Pf5 molecular orbital diagram. What is the molecular geometry of cli5 enter the molecular geometry of the molecule? Since there are six electron pairs on the central atom, then VSEPR theory tells us that the electron pair geometry is octahedral and with 5 bonding pairs and 1 lone pair, the molecular geometry is square pyramidal. Is Pentafluoride polar? So, is PF5 polar or ... PF5 has a VSEPR theory AX5 geometry so we need hybrid orbitals suitable for bonds to. 5 atoms. ns and np combinations can only provide four, so we need to use ...32 pages Does pf5 obey the octet rule Considering the tremendous variety in properties of elements and also compounds in the routine system, it is asking a good deal to expect a dominance as basic as Lewis' octet concept to be able to predict all formulas or come account for all molecular structures entailing covalent bonds. PF5 Hybridization The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3, but when it is in an excited state, the electrons from 3s orbital get unpaired. There are five half-filled orbitals: one s orbital, three p orbitals, and one d orbital.
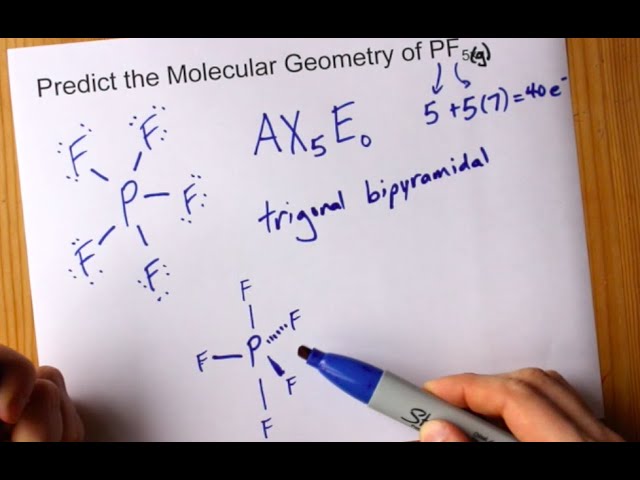
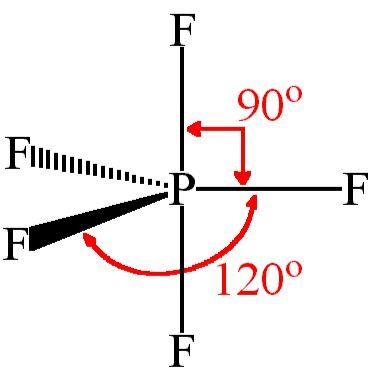
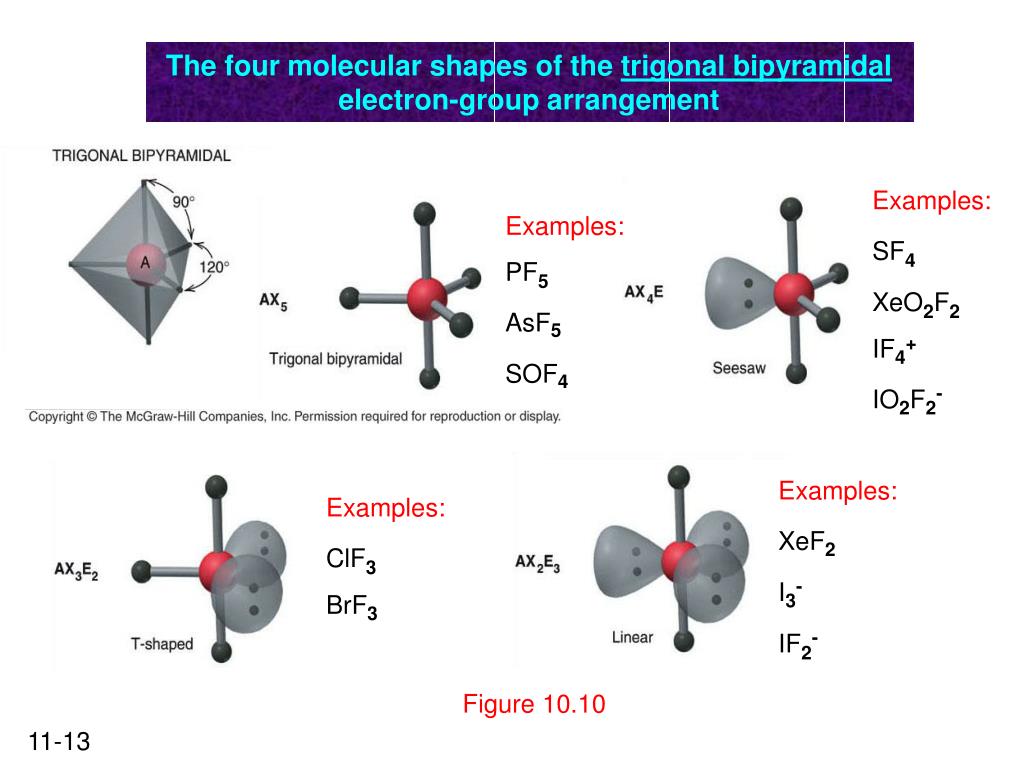
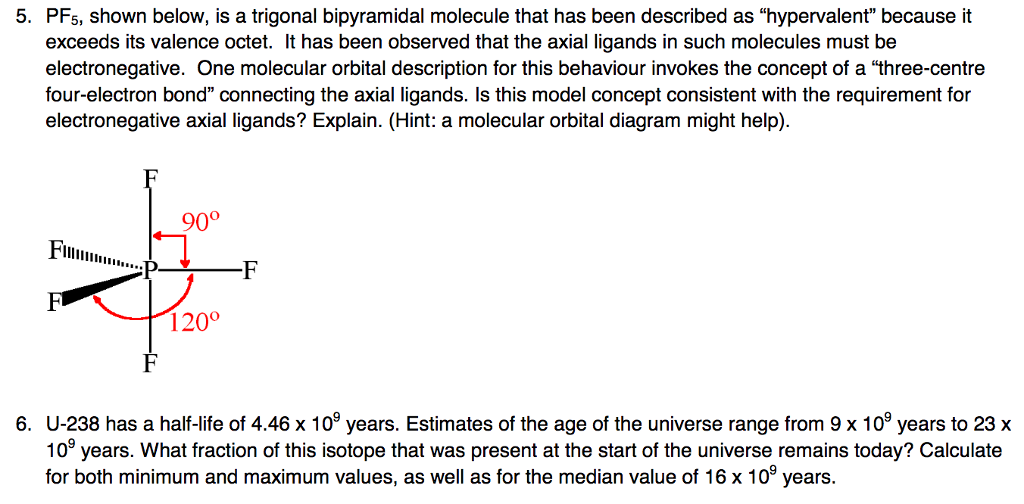
The molecular geometry of PF5, phosphorus pentafluoride, is triangular bipyramidal. The Lewis structure for bromine pentafluoride is square pyramidal. What is the hybridization and geometry of PCl5? hybridization of pcl5. It is prominent that all the bond angles in trigonal bipyramidal geometry are not identical. Describe the bonding in PF5 by deriving an MO diagram. 1. Derive the LGO's for sigma bonding in PF5. a) draw the Lewis structure and assign a point. In PF5, the Phosphorus atom contains a total of 5 valence electrons and fluorine has 7 electrons in its outermost shell. One electron is shared by each fluorine atom to complete its octet. The elements present in the third period comprise of d orbitals along with s and p orbitals in the periodic table. SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ...
Answer: The octet rule is just a handy rule of thumb, it can be a useful first guide for sorting out chemical formulations - however - atoms do not have thumbs. The octect rule is a rule for atoms where the only available orbitals are s and p. Between the two there are eoght available spaces for ... A covalent bond results: a.when two ions of opposite charge collide. b.only when two atoms of the same element collide. c.when one atom donates an electron to another atom. d.when one or more pairs of electrons are shared between two atoms. e.only when a metal and a nonmetal form a bond. Orbital Theories 466 11-1 What a Bonding Theory Should Do 467 11-2 Introduction to the Valence Bond Method 470 11-3 Hybridization of Atomic Orbitals 472 11-4 Multiple Covalent Bonds 481 11-5 Molecular Orbital Theory 486 11-6 Delocalized Electrons: An Explanation Based on Molecular Orbital Theory 497 A01_PETR4521_10_SE_FM.QXD 1/16/16 12:32 PM Page x Academia.edu is a platform for academics to share research papers.
Atoms of which element, indicated by letter on the periodic table, have the orbital-filling diagram shown below? A What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) moving at a velocity of 3.0 × 107 m/s (10% of the speed of light)?
The complete sigma-only MO scheme for PF5 is shown below. With phosphorus dz2 participation, the σ a1' HOMO would be stabilized (lowered in energy) to become a ...9 pages
The phosphorus in PCl5 readily accepts electrons from other molecules. Draw molecular geometries for the reactants and the product ofthe reaction of PF5 with the Lewis base NH3. Phosphorus trichloride has a lone pair and therefore can act as a Lewis base. My answer is option 2 but the answer provided is 4 where am I going wrong.
But Nitrogen does not have vacant d orbitals but phosphorus has empty 3d orbital. So it can accept more electrons and can increase its covalency to 5 to make PF5. Why is PF5 unstable? Phosphurus can form bonds to five ligands, as in PF5 or PCl5, by using its d-orbitals. F and Cl have p-orbitals, H do not.
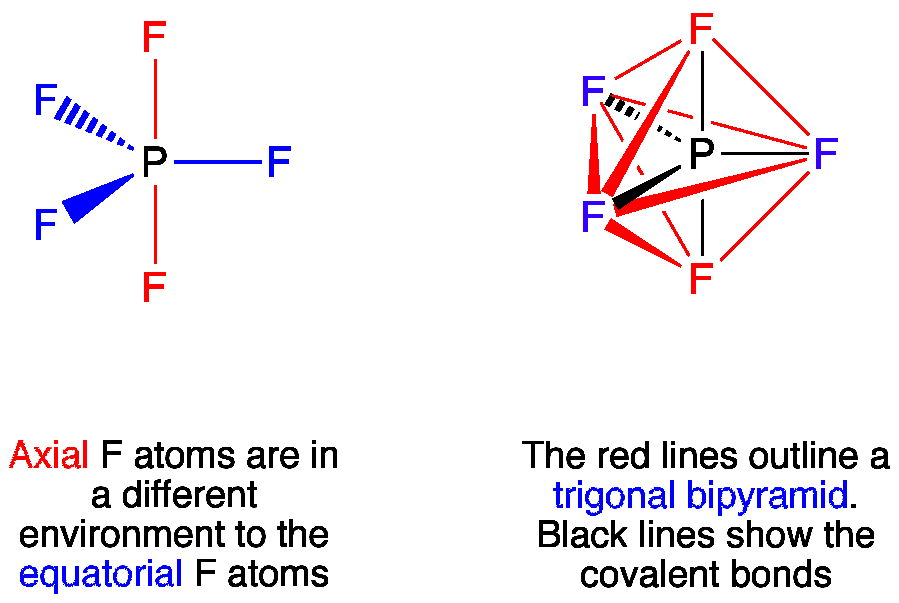
In PF5 phosphorus has 5 bond pair and 0 lone pair so steric nois 5. Thus it has two distinct types of PF bonds axial and equatorial. A quick explanation of the molecular geometry of PF5 including a description of the PF5 bond anglesLooking at the PF5 Lewis structure we can see that there.
What is PF5 compound name? Phosphorus fluoride (PF5) Phosphorus, fluoride, penta-Is scl2 a bent molecule? Conclusion. Sulfur dichloride is a bent shaped molecule due to the presence of 2 lone pairs on the sulfur atom. The sulfur atom is the central atom surrounded by two chlorine atoms with a bond angle of around 103 degrees.
I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 + I- —-> I3-. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings.
If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Yes, ethyl acetate (or ethyl ethanoate) is a Lewis base since it has the ability to act as an electron-pair donor. The classification into hard and soft acids and bases ( HSAB theory ) followed in 1963. Phosphor(III)-iodid, Oxidationsstufe (V): chemistry, polar or nonpolar. Im Vergleich zu ...
SF4 Molecular Geometry. ... Hence the sulfur atom uses five hybridized orbitals, one 3s orbital, three 3p orbitals, and one 3d orbital. This arrangement of electrons around the atom and hybridized orbitals leads to the sp3d hybridization. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom.
In PF5, there is a lone pair of electrons on phosphorus where as in IF5 there are no lone pairs on iodine. As a result, the molecular shape of PF5 is square pyramidal and IF5 is trigonal bipyramidal. As a result, the molecular shape of IF5 is octahedral and PF5 is trigonal bipyramidal. How many bonds does C have in CH3OH? three bonds
Molecular Orbitals diagram of Phosphorus Trifluoride (PF3) molecule. The molecular orbital diagram helps with determining how chemical bond formation is taking place. Also, it helps with figuring out how mixing and overlapping have taken place to produce four new hybrid orbitals.
1 day ago · A metal such as iron has metallic bonding. The lobes of the p orbitals are 90E and 180E apart from each other. Table Ionic and Covalent Compounds Name: KEY!! 1. Ionic, Molecular, or an Acid (Honors Chemistry) Write which type of compound it is, whether the compound is ionic, molecular, or an acid.
A square planar complex is formed by hybridisation of which atomic orbitals. #109. In benzene, all the six C - C bonds have the same length because of. #110. The bond energies of H - H and Cl - Cl are 430 kJ mol−1 and 242 kJ mol−1 respectively, ∆Ht for HCl is 91 kj mol. The bond energy of HCl will be. #111.
Nov 29, 2021 · What is the maximum degeneracy of its molecular orbitals? Which P3p orbitals contribute to a molecular orbital of this degeneracy? Question: Determine the point group of the PF5 molecule (use vsepr). 1-1-1 1 B 2-1 1-1 1 B 1-1-1 1 1 A 2 1 1 1 1 A 1 σ ’ v (yz) σ v (xz) C 2 E C 2V Representation PS5 has a powerful 8-core AMD Zen 2 processor, 10.
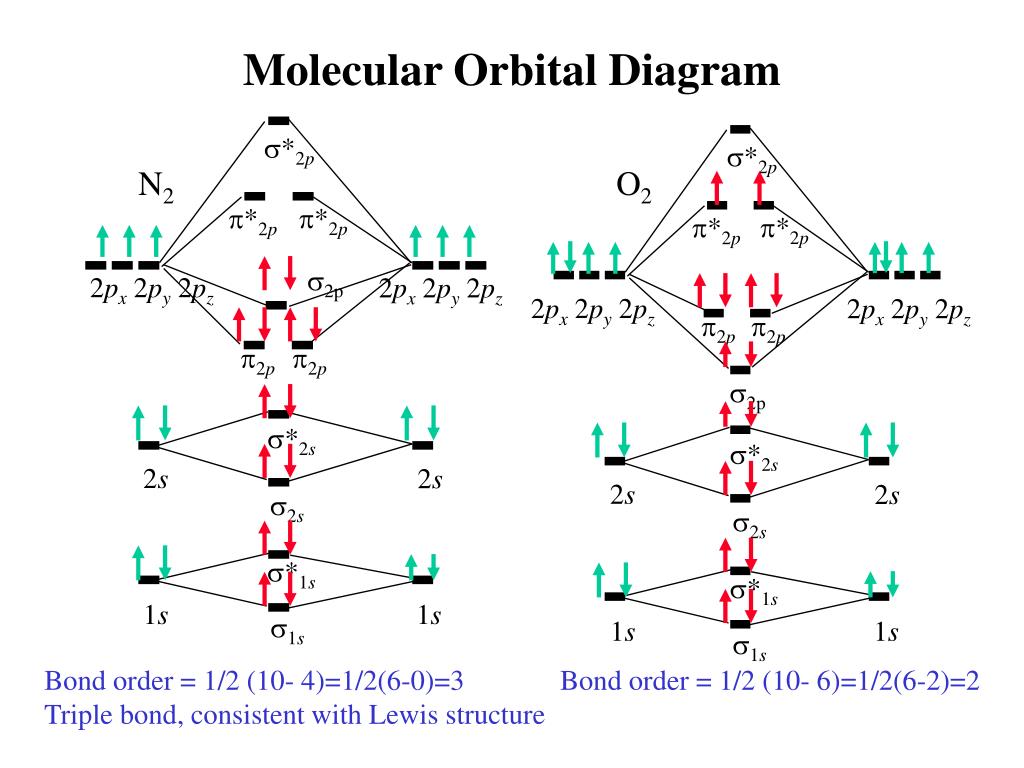
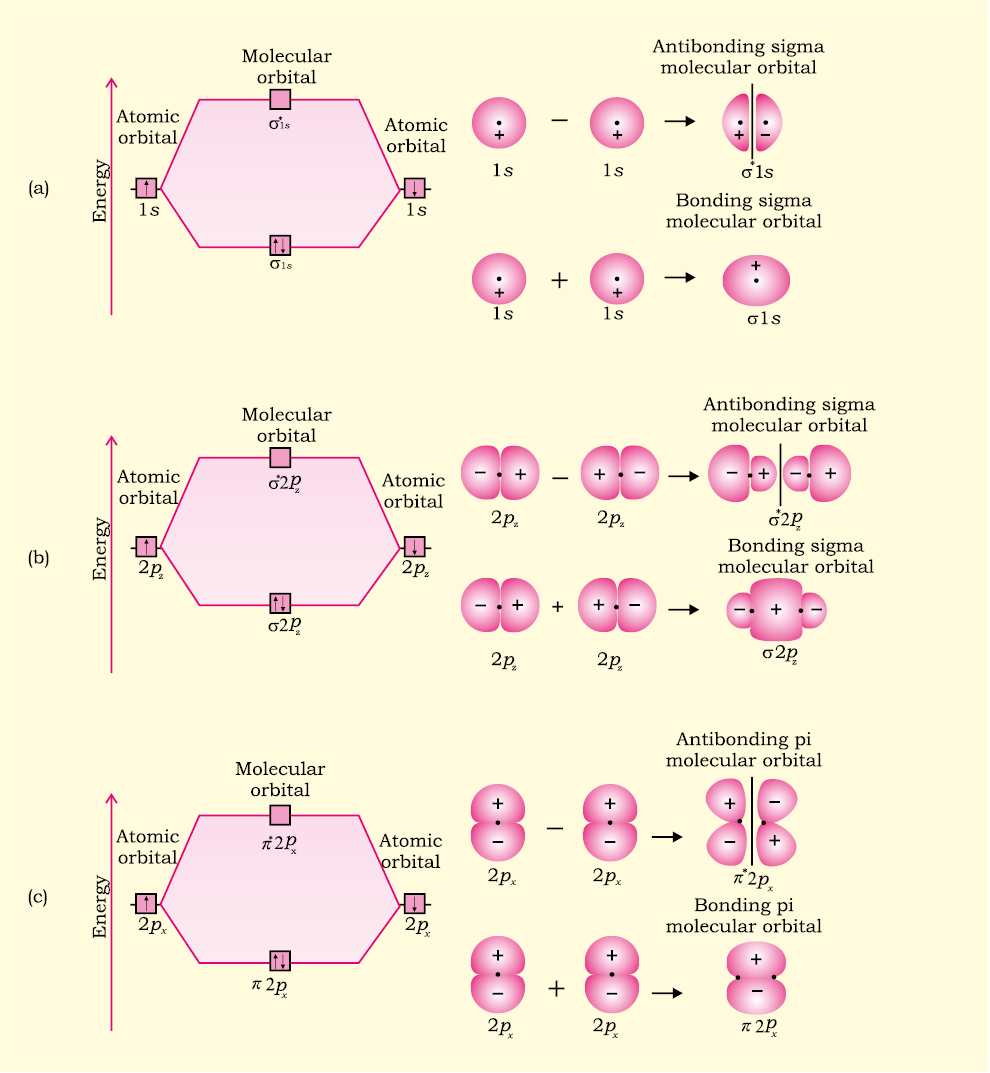
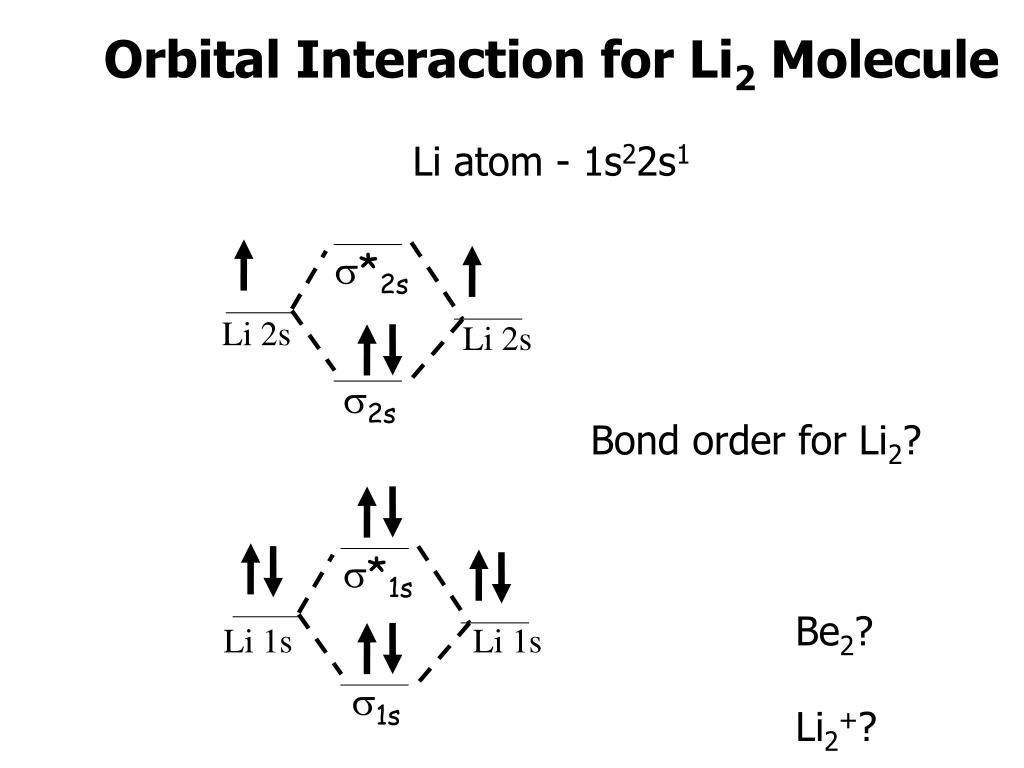
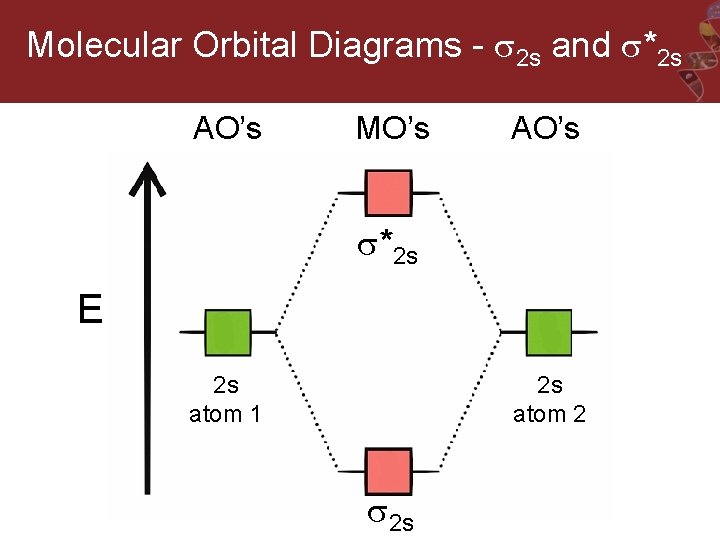
Molecular hydrogen consists of two hydrogen atoms, each containing 1 electron in a 1s atomic orbital. Therefore, the molecular orbital diagram for H2 will have 2 electrons, both of which are placed in the lowest energy sigma bonding molecular orbital.
Q.11. Assertion : Bonding molecular orbital has greater stability than corresponding antibonding molecular orbital. Reason : The electron density in a bonding molecular orbital is located away from the space between the nuclei while in antibonding molecular orbital it is located between the nuclei of the bonded atoms.
In phosphorus pentafluoride (PF5), phosphorus is the central element. The phosphorus atom has 5 valence electrons in its outermost shell (3s2 3p3) and so it is capable of forming 5 bonds with 5 atoms of fluorine to form PF5. But the hydrogen atom is a one-electron entity as it has only one electron (in the 1s orbital) around its nucleus.
C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms and two hydrogen atoms.
by R Hoffmann · 1965 · Cited by 329 — a molecular orbital description which is (1) simple, ... Two interaction diagrams for the construction of the molecular orbitals of Dah PH5 ... PHs and PF5.12 pages
Construct the valence molecular orbital diagram for PL5 where L is a σ-bonding ligand. Sigma bonding ligands contribute sp type hybrid orbitals, which "look" ...4 pages
The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals. What is the hybridization of pf5? The hybridization is sp3d hybridization and phosphorous atom forms five sp3d hybrid orbitals. Five hybrid orbitals will be used to form bonds with five fluorine atoms.
Pi5 has been given the systematic name of α-KTx 24.1, being the first member in the 24th subfamily of α-KTx toxins. The Pi5 peptide has a molecular weight of 3334.00 Da. Why does PF5 exist but not PH5? Answer. Answer: PH5 is formed by the overlap of d orbitals with Sp3d hybridisation.
Academia.edu is a platform for academics to share research papers.
Nov 5, 2013 — bond (VB) theory and molecular orbital (MO) theory. ... This approach has been used successfully to describe the bonding in PF5 (Scheme.4 pages
PF5 SF6 elements from the third period on have unfilled d orbitals that can acomodate additional electrons. Case they do not exceed the octet rule. With this model we can draw a series of resonance structures as shown below for PF5. A On your work draw the Lewis structure of phosphorous pentafluoride and label the formal charge of any atom with ...








0 Response to "37 pf5 molecular orbital diagram"
Post a Comment