37 molecular orbital diagram of h2
3 Feb 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ...
Hi guys, I noticed that recently several users have posed queries about H3 Chem. I'd be happy to answer any questions regarding the subject! You can also ask about other H3s, I'll answer based on my friends' and my own knowledge as well. Other users please feel free to contribute! Also below is a comment that I made previously regarding whether one should take H3 Chem. You can take a look if you want. I'm going to list out 4 reasons why H3 might be helpful for you (at least, imho). If they don...
Good morning, I'm looking for a way to compute molecular orbital diagrams using fragments or different molecules. I mean something like the diagram linked [here](https://commons.wikimedia.org/wiki/File:H2O-MO-Diagram.svg), where, instead of considering the H2 fragment and the oxygen atom I could put two fragments chosen by me or two molecules. I searched but, at the moment, I didn't find anything useful. I can use Gaussian or Orca for the calculations so if it was possible to obtain such dia...

Molecular orbital diagram of h2
In this video, we take a detailed look at the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this v...
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
Hello guys ! I have my finals in less than a month and I'm definitely stuck on this part of the program; I would like someone trying to explain me how you actually make a molecular orbital diagram through a relatively simple example like H2 or less simple like O2 in the form of an infographic, with a lot of arrows in it or something in this kind :) This would really help me, thanks in advance for your time ! Have a wonderful day !
Molecular orbital diagram of h2.
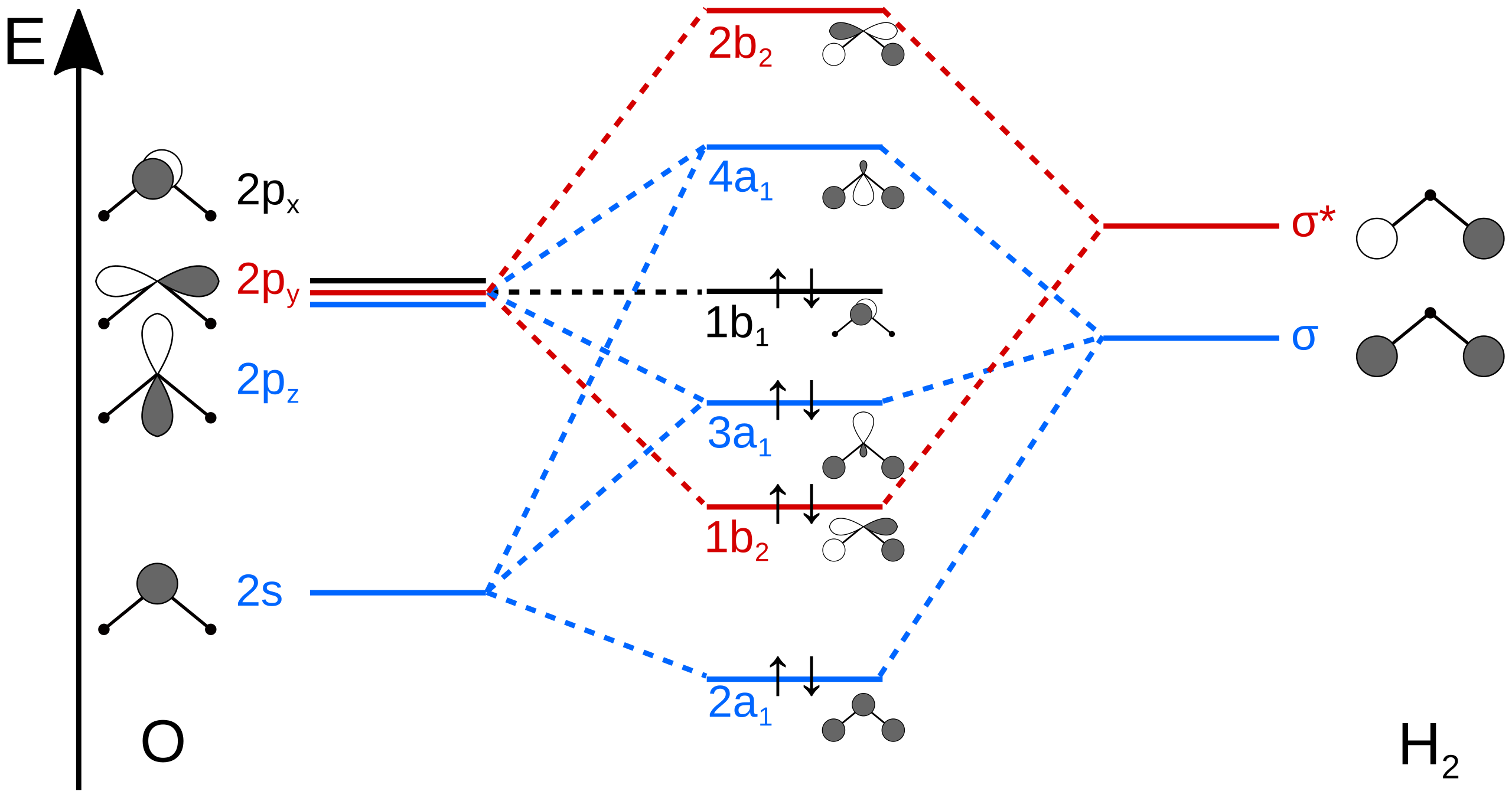
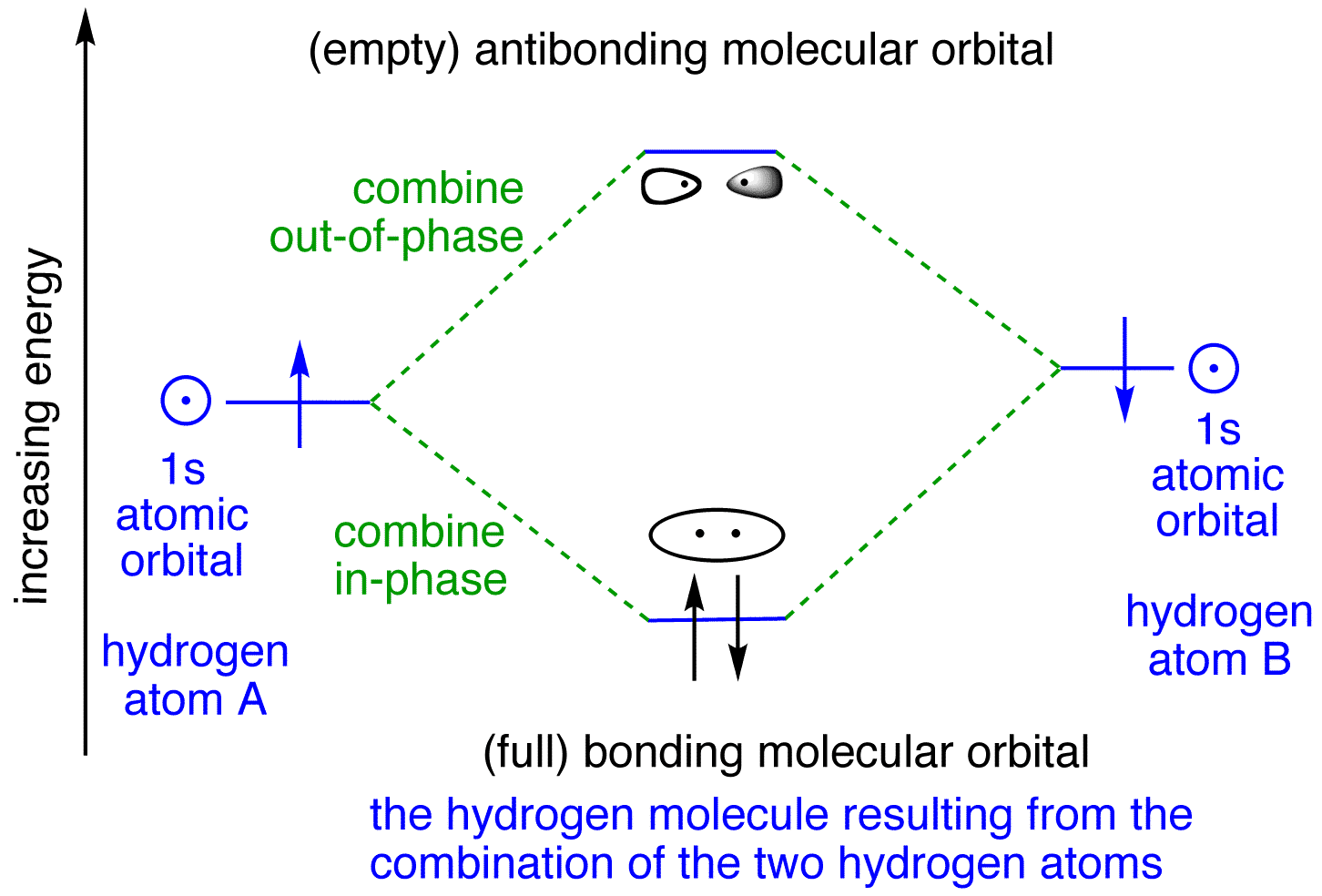
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
Nov 08, 2021 · Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2 - is to be drawn. According to MOT number of atomic orbital s combined is equal to total number of molecular orbital s formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... 3:55This video discusses how to draw the molecular orbital (MO) diagram for the H2 (2+) molecule.
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
22 Dec 2020 · 1 answer1. Electronic configuration of H atom 1s ; 2. Electronic configuration of H, molecule – σ1s1 Bond order ; 3. Molecule (H2) has no unpaired ...
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is given by: gs ψψ αβ βα++ (1) (2) (1) (2) (2) (1) / 2[ ] [ ][ ] 12 1 ψψ ψ(1) (2) 1 (1) 1 (1) 1 (2) 1 (2) ( , )= ss s s rr AB A B++≡ GG with: ++2(1 )+S
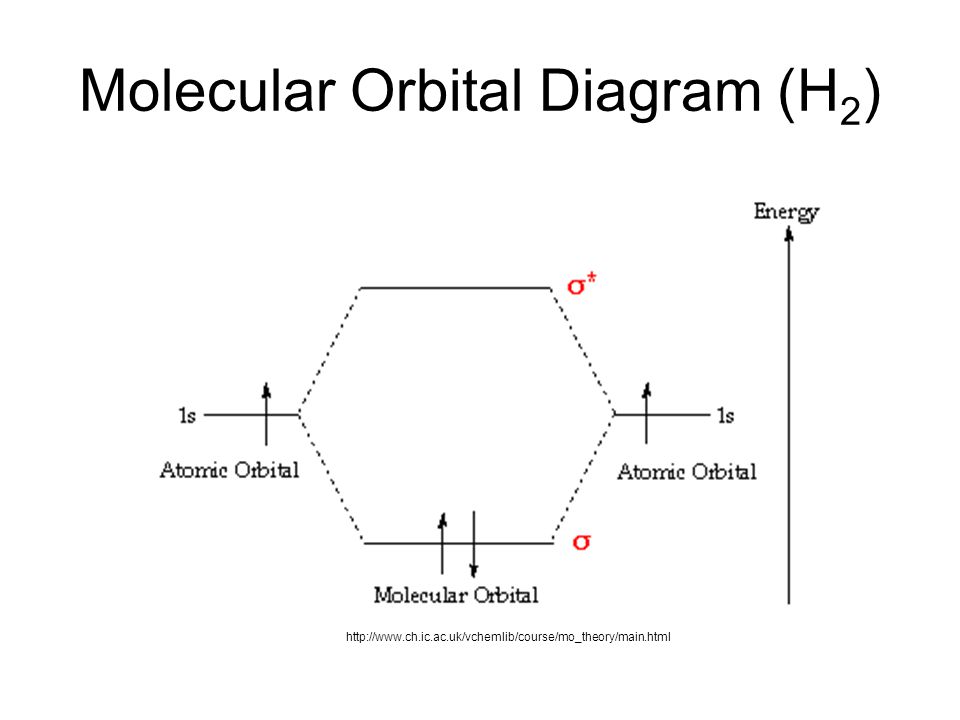
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
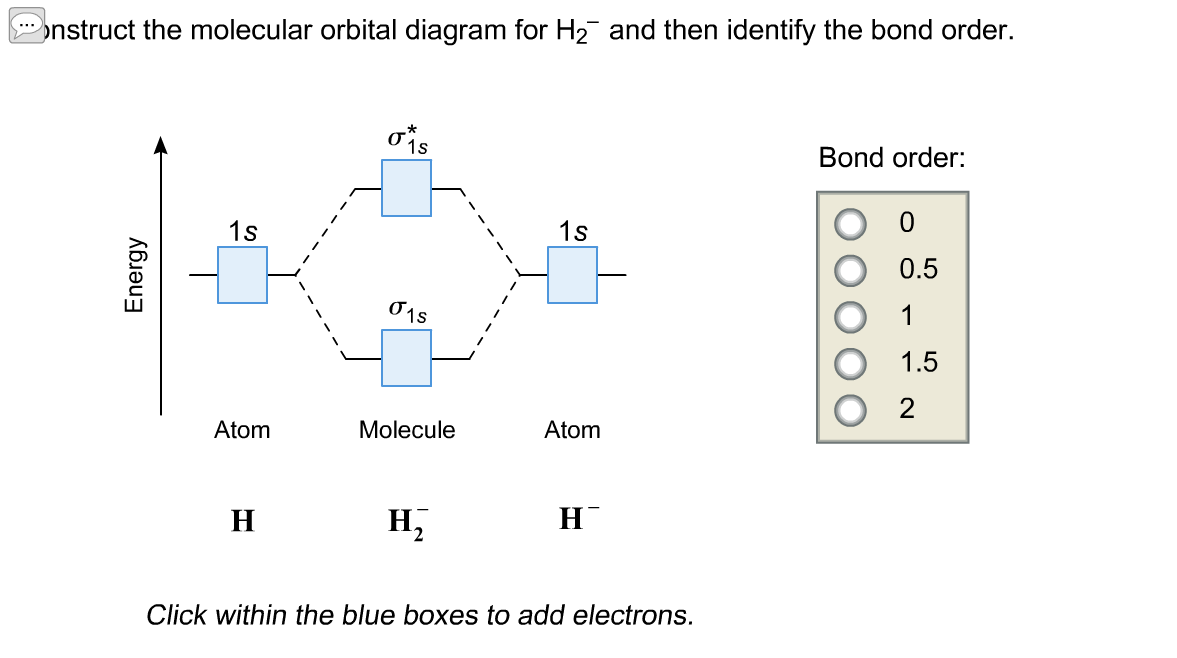
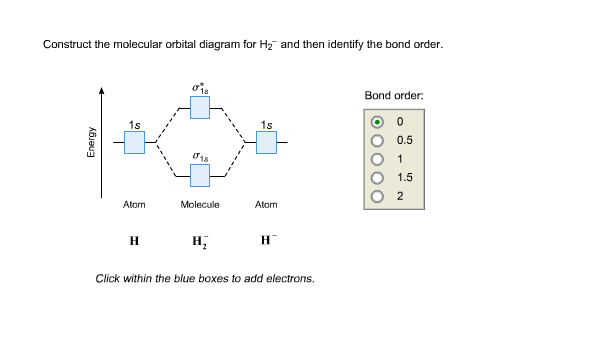
Dec 15, 2018 · Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.










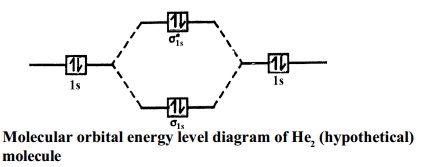


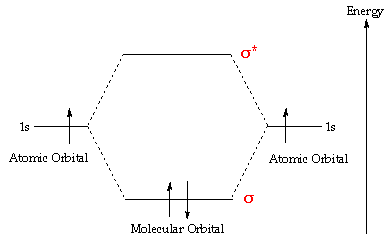

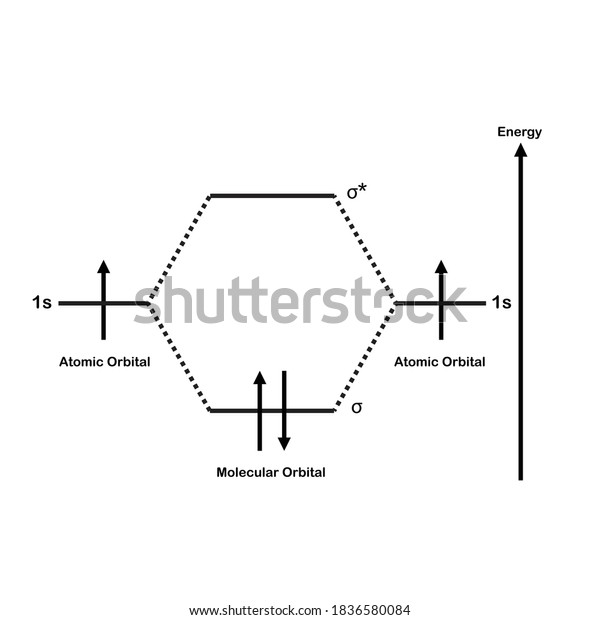

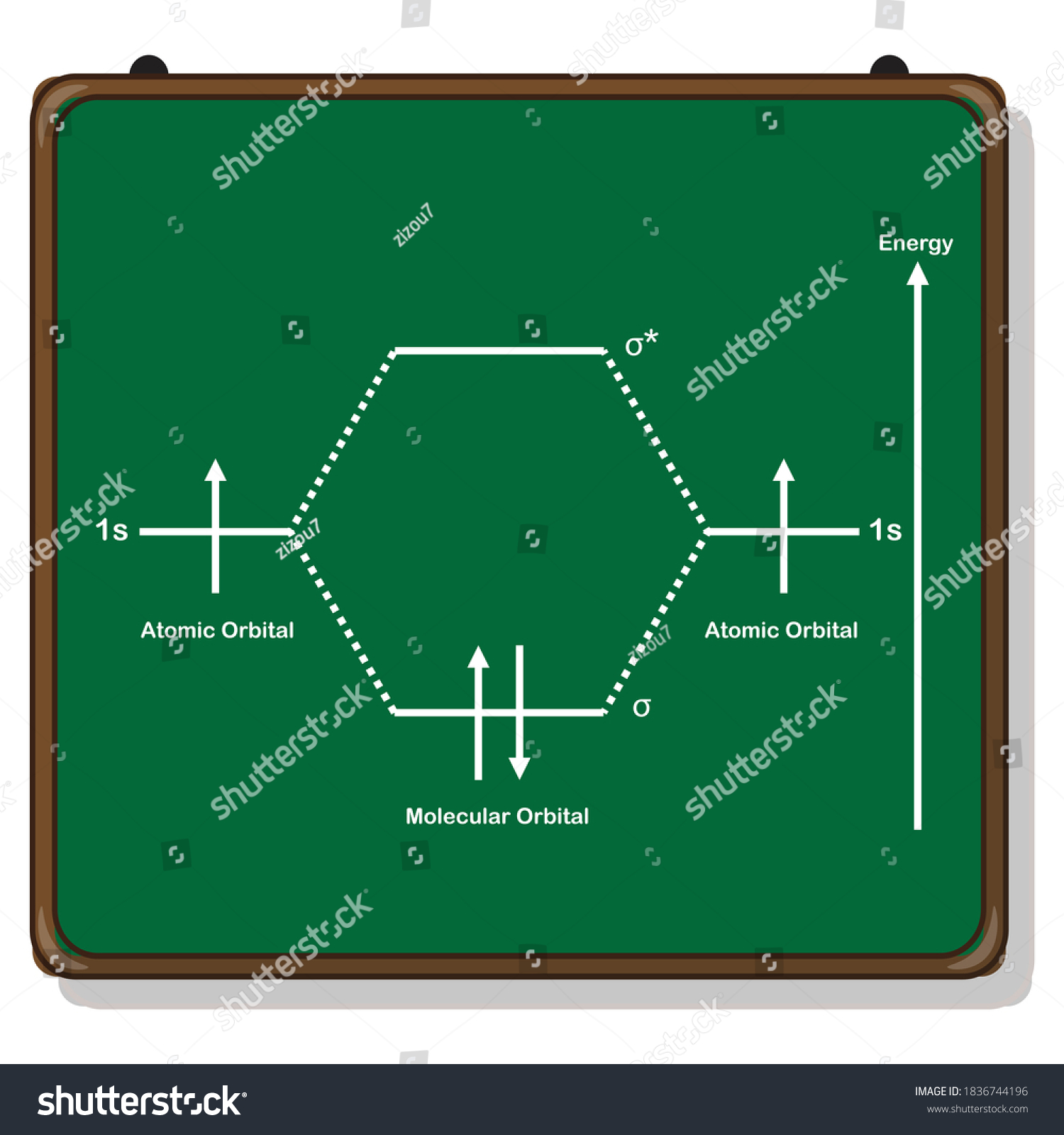





0 Response to "37 molecular orbital diagram of h2"
Post a Comment