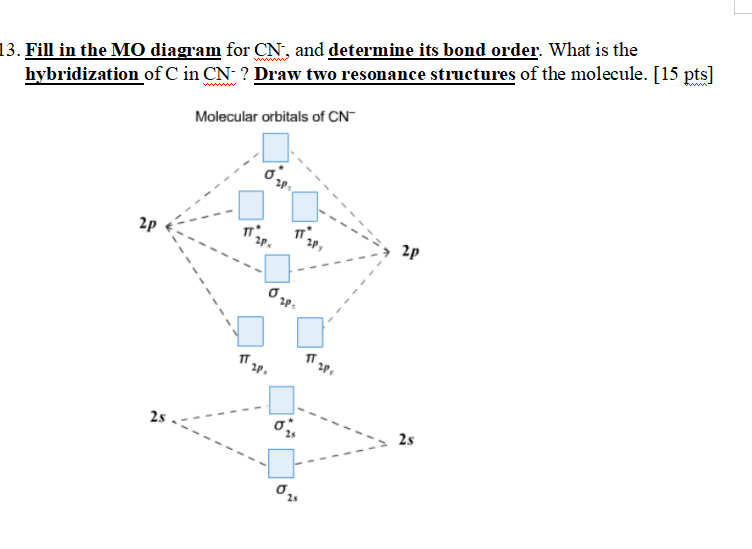
37 molecular orbital diagram for cn-
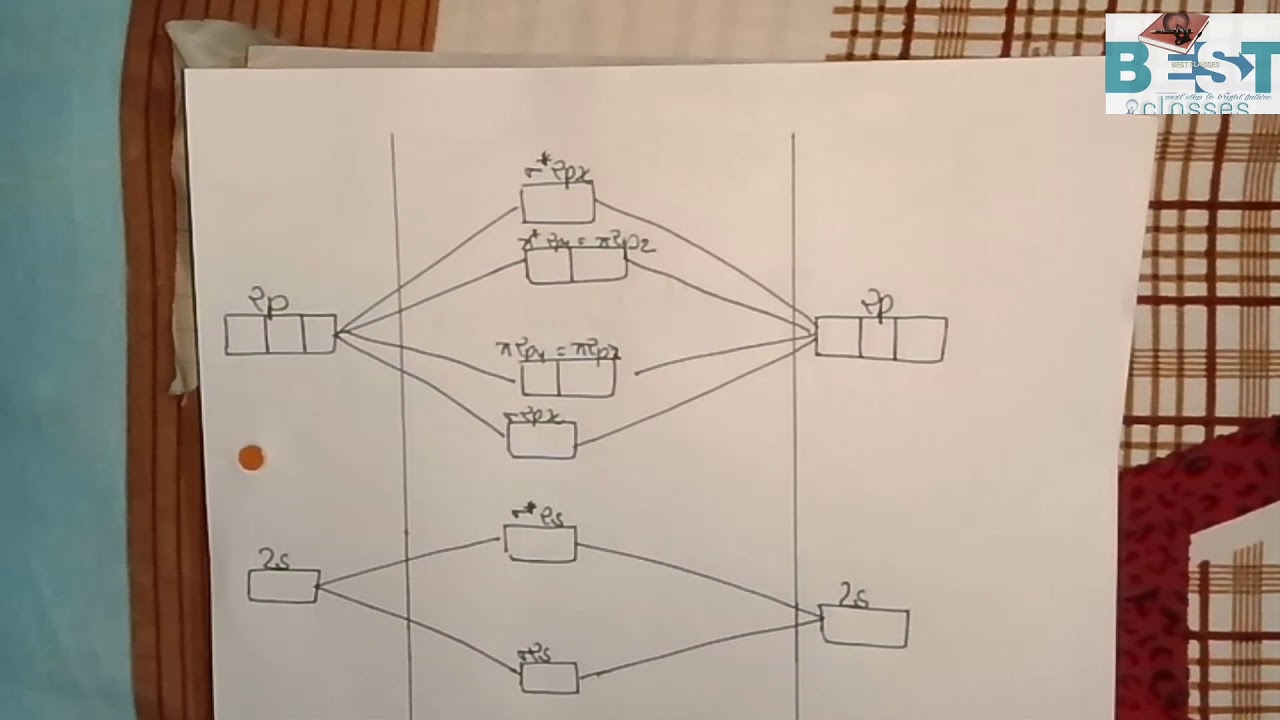
The molecular orbital diagram of CN molecule is shown in the following figure: Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition. Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory) Reviewed by تعرف على علم الكيمياء on 5/20/2019 Rating: 5 Answer (1 of 5): The total number of electron of CN- ion is ( 6+7+1) = 14 . According to molecular orbital theory, the electronic configuration of CN - ion is as follows, From the above electronic configuration , it has been found that , the number of bonding electron is 10 and the the number of...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...

Molecular orbital diagram for cn-
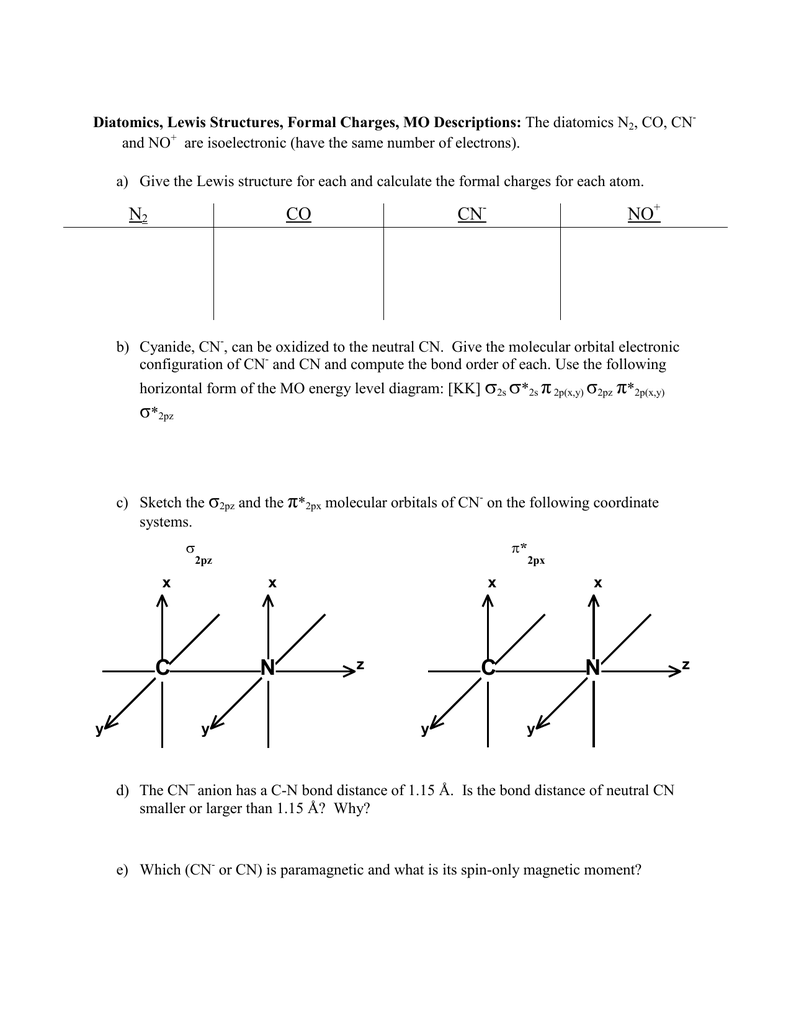
Problem: Use the molecular orbital diagram to figure out the electronic configuration for CN. Which of the following statements is correct?a) CN is diamagnetic.b) CN− is paramagnetic.c) If an electron is removed to give CN+, the bond order increases.d) The π*2p orbital is the highest energy orbital containing an electron in CNe) If an electron is added to give CN−, the bond length decreases. Problem 1: (a) Prepare the MO diagram for the cyanide ion, CN −.Use sketches to show clearly how the atomic orbitals interact to form MOs. (b) What is the bond order, and how many unpaired electrons does cyanide have? (c) Which molecular orbital of CN − would you predict to interact most strongly with a hydrogen 1 s orbital to form an H-C bond in the reaction: CN − + H + → HCN? MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
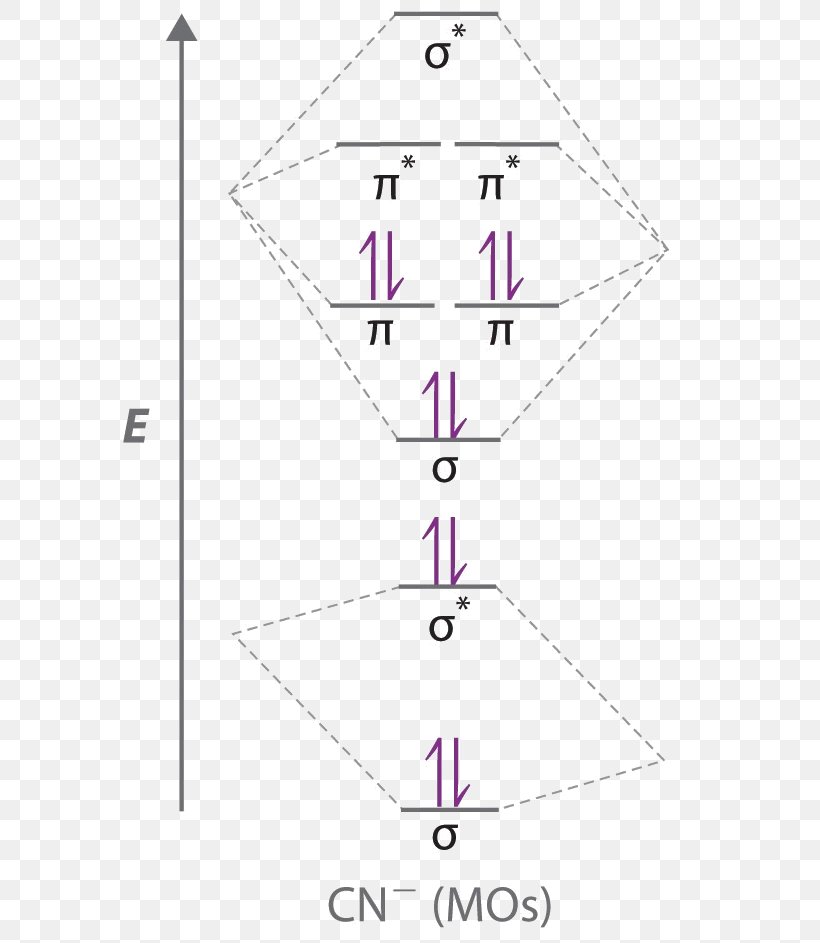
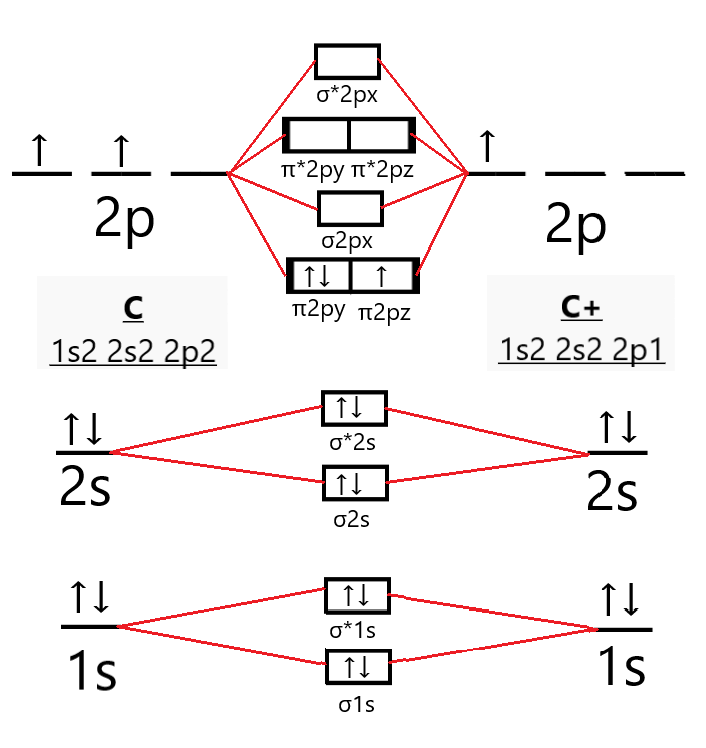
Molecular orbital diagram for cn-. This video is about MO Diagram #3 - CN- The molecular orbital configuration of C N + is K K σ (2 s) 2, σ ∗ (2 s) 2, π (2 p x ) 2, π (2 p y ) 2. Bond order is 2 . All the electrons are paired and ion is diamagnetic. Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ... Tania Havenga. University of South Africa. Here is the MO for CN-, just take away a single electron from the MO since CN is neutral. Vijayta Gupta is right, the N atom is lower in energy. http ...
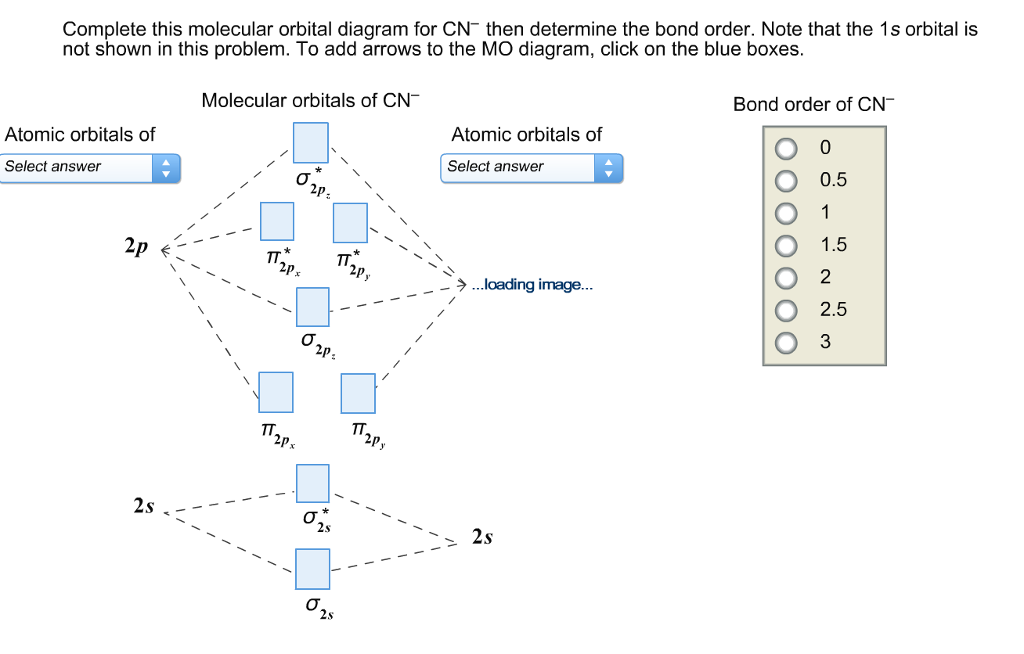
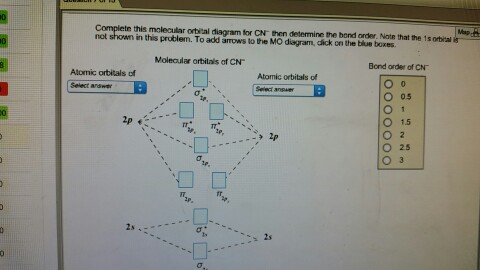
Cyanide Molecular Orbital Diagram. MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of. 6 answers[math]\text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2}[/math] Here is the molecular orbital diagram of CN-: There ... together to produce a sigma molecular orbital [σ = (1sa + 1sb)]. Since the electrons in this orbital are more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This ...
How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... FREE Expert Solution. We're being asked to complete the molecular orbital diagram of CN- and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate the total valence electrons present. Step 2: Fill the molecular orbitals with electrons. Step 3: Determine the bond order. Step 1: Calculate the total valence ... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
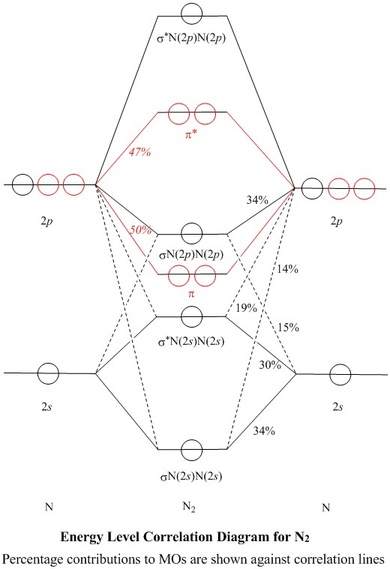
uh, molecular orbital represents. So they have, like, behavior off electron. They're formed into the linear combination for atomic orbital spawned in there at them. So fair manicure. Um, the the electronic configuration off carbon and nitrogen is carbon is one is to toe to toe pay two and a nitrogen is 12 tourist to and to be three. So the total electron in the Hadron ah, hit your own nuclear ...
Molecular orbital Diagram for Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory ...
• The molecular orbital energy level diagrams for H 2, H 2 +, H 2 ... The extra electron in CN-occupies a σ-orbital. This is a bonding orbital and so CN-has more bonding electrons than CN and would be expected to have a stronger bond as a result. Equivalently, bond order can be used to rationalize the bond strength: ...
Molecular Orbital Mixing. More detail was added to this answer in response to input and questions from students in the class of 2008. If you have suggestions or contributions please e-mail me. First of all while the stage 1 (pre mixing diagram) of diatomics is very easy to produce, the mixing in diatomics is very difficult to evaluate because ...
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital and the other is called an anti-bonding molecular ...1 answer · Top answer: Concepts and reason • The molecular orbital theory explains the bonding in terms of the combination and organization of atomic orbitals of an atom which ...
Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3.
Molecular orbital Diagram Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory heteronuclear ...
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion).
Molecular orbital diagram for cn. Note that the 1s orbital is not shown in this problem. Note that the 1s orbital is not shown in this problem. Maybe the best example for this is the mentioned ce cn and obviously the isoelectronic ceco. To add arrows to the mo diagram click on the blue boxes. Vijayta gupta is right the n atom is lower in energy.
In the second diagram only sigma bonding is considered and it shows the combination of the metal 3d, 4s and 4p orbitals with OCCUPIED ligand group orbitals (using 1 orbital from each ligand). The result is that that the metal electrons would be fed into t2g and eg* molecular orbitals which is similar to the CFT model except that the eg orbital ...
Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Problem 1: (a) Prepare the MO diagram for the cyanide ion, CN −.Use sketches to show clearly how the atomic orbitals interact to form MOs. (b) What is the bond order, and how many unpaired electrons does cyanide have? (c) Which molecular orbital of CN − would you predict to interact most strongly with a hydrogen 1 s orbital to form an H-C bond in the reaction: CN − + H + → HCN?
Problem: Use the molecular orbital diagram to figure out the electronic configuration for CN. Which of the following statements is correct?a) CN is diamagnetic.b) CN− is paramagnetic.c) If an electron is removed to give CN+, the bond order increases.d) The π*2p orbital is the highest energy orbital containing an electron in CNe) If an electron is added to give CN−, the bond length decreases.












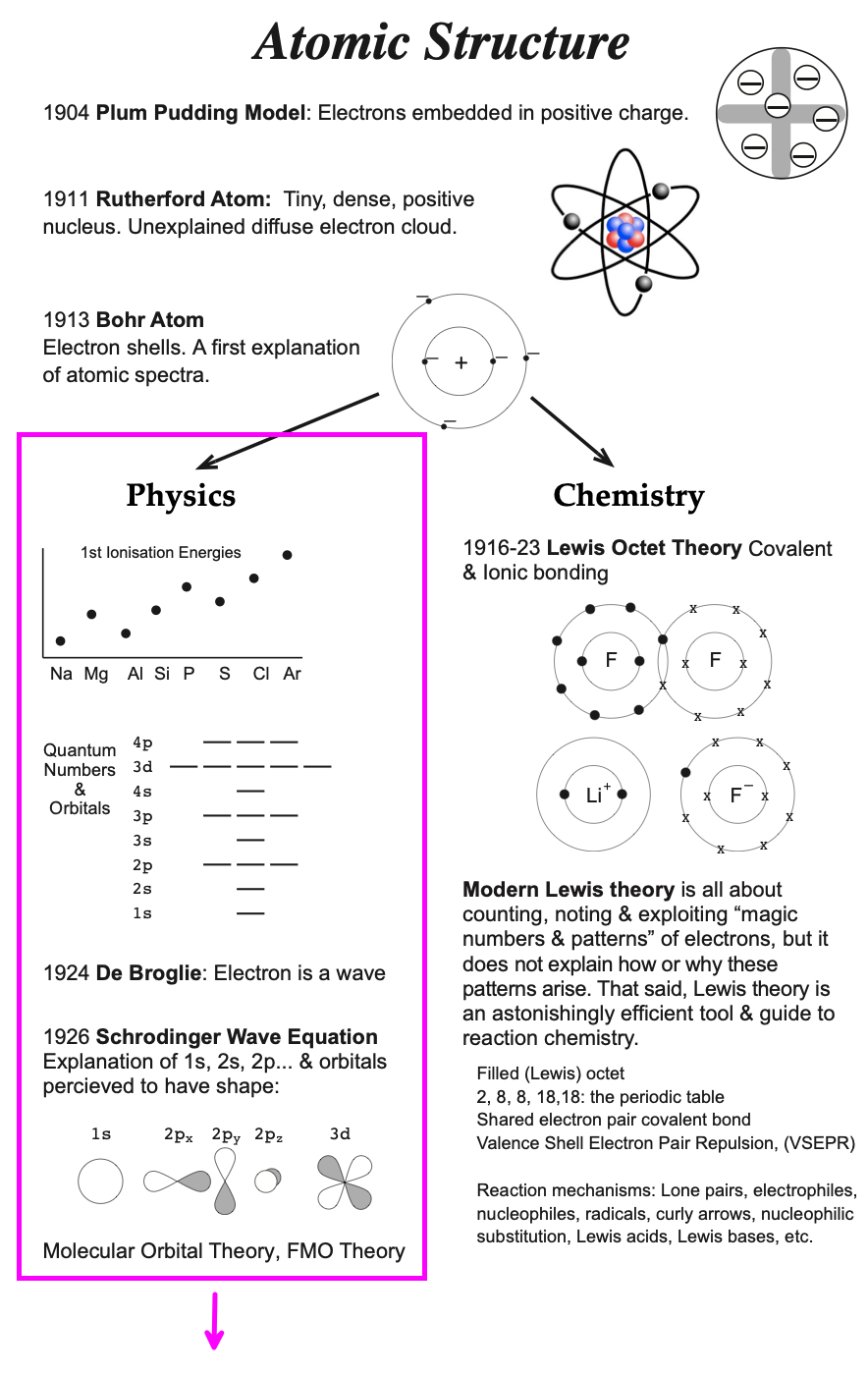
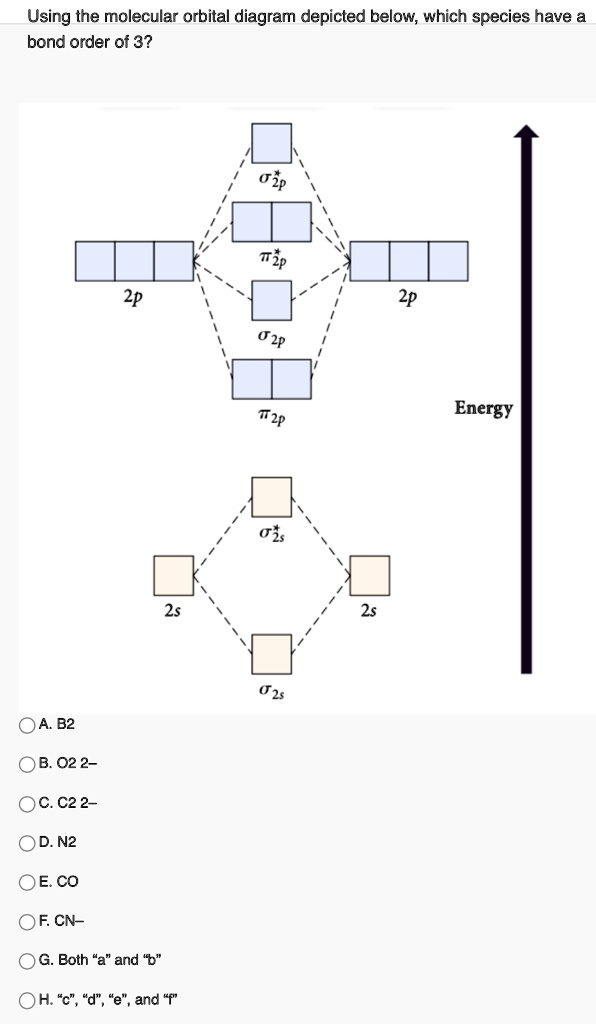
0 Response to "37 molecular orbital diagram for cn-"
Post a Comment