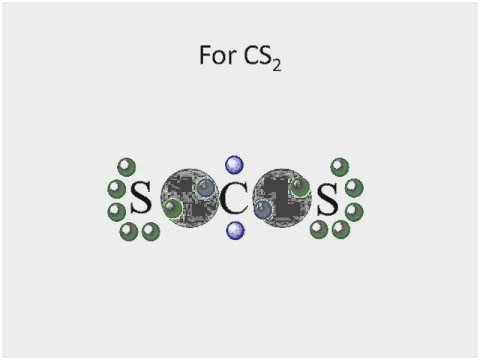
37 lewis dot diagram for cs2
6 Nonzero formal charges are indicated for each atom in the structure once the total number of electrons is correct. Schiferl D, Review of Scientific Instruments, 1977, 48, 24. The Lewis acidity of bismuth(III) halides: a DFT analysis. STUDY. 5. Created by. Both molecules are pyramidal (C3v symmetry), and their geometries are characterized by the following bond lengths (rg) and bond angles ...
Lewis dot structure. The Lewis dot structure for Strontium Sulfide is simply Sr-S. The S atom has 3 nonbonding electron pairs around it. Every element in the second group (column) has 2 dots in the electron dot structure (also known as the lewis dot structure): Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium.
1 answerWe're being asked to draw a Lewis structure for CS2. To answer this problem, we must: Step 1. Find the center atom of this compound.

Lewis dot diagram for cs2
The Arsenic has 10 valence electrons, but that's OK. What is the Lewis structure and 3-D structures of these elements: OF 2. GaI3 5. General rules for drawing Lewis structure. 1. (previous page) 2-bromopropane-2D-Lewis.png 1,100 × 761; 30 KB. There are a total of 40 valence electrons in AsF 5. Then we'll go around the outside and complete the octets for the Fluorine. The following is a list ...
Lewis dot structure for: CH3Cl; H2Te; NH3; TeI2; C2H6; C3H6; C2H5Cl; HCN; CS2; C2H5OH; N2; H2NCH2Br; The symbol Sb stands for stibnum or stibnite. Mol mass of BeCl2 = 2* 35.4 (mol mass of Cl) + 1 * 9 (mol mass of Be) = 79.92 g/mol. B Sulfur hybridizes its 3s and 3p orbitals, to produce four sp 3 hybrids.
Lewis Structure of Dichlorine Monoxide (OCl2) Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. The structure determines how sharing of the valence electrons is taking place and whether a single, double or triple bond is forming.
Lewis dot diagram for cs2.
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
Jan 06 2021 08:41 PM. Lewis dot structure for: CH3Cl; H2Te; NH3; TeI2; C2H6; C3H6; C2H5Cl; HCN; CS2; C2H5OH; N2; H2NCH2Br; The correct formula of the compound whose name is hexaamminechromium (III) nitrate is …. The Be-F Bond In BeF, Is O 180 109.5 O < 109.50 120 Nonpolar Polar The Molecule Bef, Is Nonpolar Polar .
Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube . How To Draw The Lewis Dot Structure For Caf2 Calcium Fluoride Youtube . Cacl2 Lewis Structure How To Draw The Lewis Dot Structure For Calcium Chloride Youtube . A Step By Step Explanation Of How To Draw The Icl3 Lewis Structure Youtube .
It is a toxic compound but is used in several industries. Write the Lewis dot symbol for. A N2O B CS2 C PH3 D CCl4 E NO2. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3. Excerpt from ERG Guide 129 Flammable Liquids Water-Miscible Noxious.
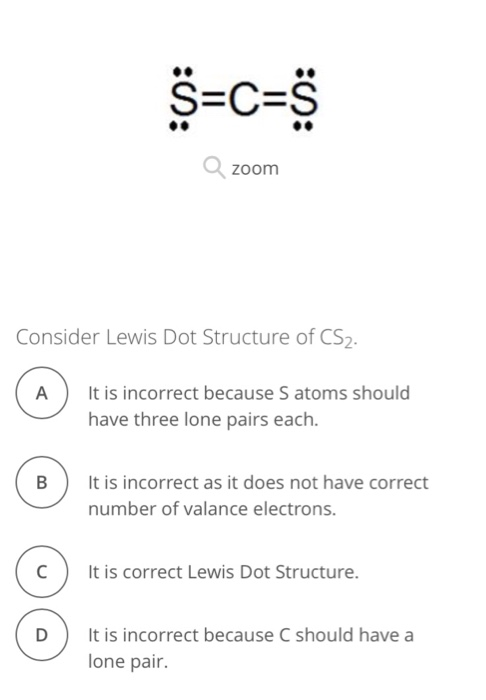
Jun 11, 2020 — The lewis dot structure for CS2 also predicts many of the properties of the molecule. Due to the presence of large sulfide atoms over for ...
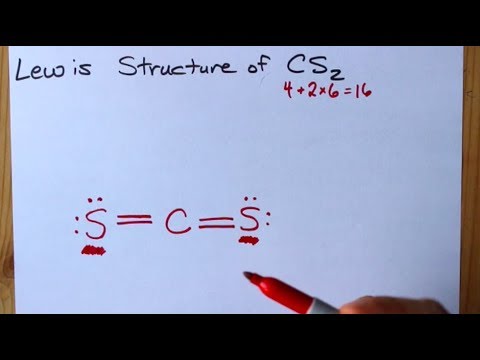
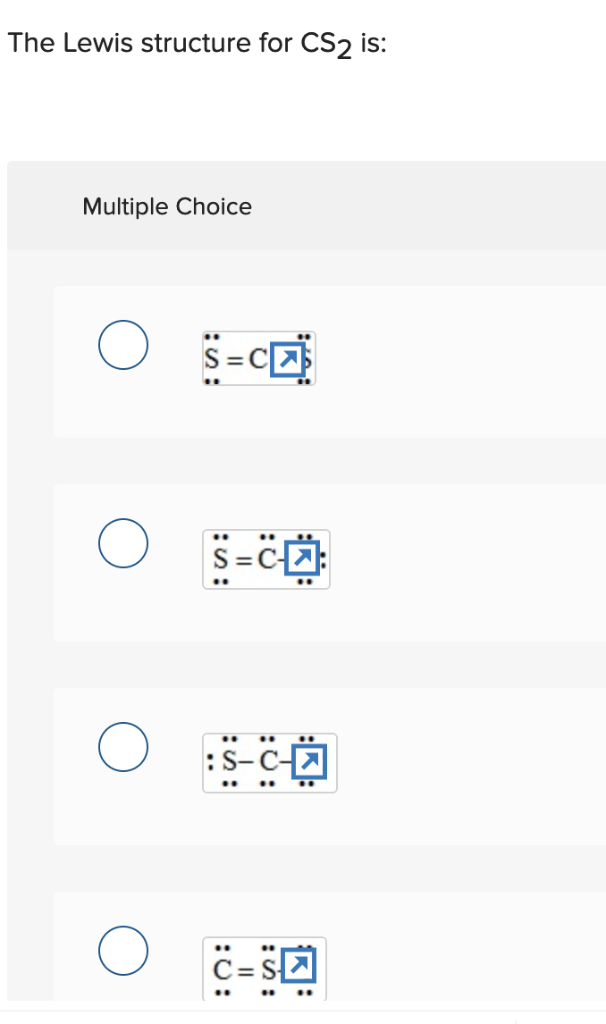
The Lewis structure for CS2 requires you have double bonds between the Carbon (C) and Sulfur atoms in order to fill the octet of Carbon.Nov 24, 2013 · Uploaded by Wayne Breslyn
masc. proper name, Anglo-French form of French Louis (see Louis).
"worthless," 1711, from adjectival phrase (see good (adj.)).
BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
To Draw Co2 Lewis Structure Follow The Steps. This structure helps in knowing the arrangement of electrons in the molecules and the shape of the molecule. Lewis dot structure is a pictorial representation of. Carbon is the least electronegative atom and goes in. Struktur lewis cs2. Struktur Lewis dari suatu molekul adalah cara.
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881).
Lewis Dot Structures Defined. In science, we often use diagrams and shorthand notation to better understand a particular concept. One example of this is a Lewis dot structure.
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
Step-3: Lewis dot Structure for CS2 generated from step-1 and step-2 — The electron dot structure of the CS2 molecule is also known as the CS2 Lewis ...
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.
1740, "mark with a dot or dots," from dot (n.). Sense of "mark or diversify with small, detached objects" is by 1818. Sense of "put a dot over (the letter i)" is by 1833. Related: Dotted; dotting. Dotted line is by 1690s.
A Lewis structure is a pictorial diagram showing how electrons get distributed around an atom. Lewis structures are drawn to help one understand or predict how many types of bonds can be formed around a given atom. The drawing is started by determining types of covalent bonds that are formed after combining atoms.
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
The CS2 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CS2 molecule. -the reactivity of a molecule and how it might interact with other molecules. Join Login 11th Chemistry Chemical Bonding and Molecular Structure.
Lewis Dot Structure For Cs2. ... Lewis Structure Of Cs2. Indicate The Electron Pair Geometry And The Molecular Geometry For Each Of The Six Compounds * Please keep in mind that all text is machine-generated, we do not bear any responsibility, and you should always get advice from professionals before taking any actions ...
The Lewis structure of carbononitridic chloride, or NCCl is as follows: A N atom is triple bonded to a C atom. When calcium loses its two valence electrons to become an ion, the Lewis structure shows it with no dots (electrons). …. You also know that Cl is a group 7 and has 7 valence electrons. The molecular formula for calcium chloride is CaCl2.
The molecular geometry of cs2 is linear with symmetric electron region distribution around the central atom. The electron-pair geometry of CS2 is linear because the Lewis structure is SCS. Carbon disulphide C S 2. Double bonds act as one electron pair to help determine electron-pair geometries of molecules according to VESPR.
"pattern consisting of dots of uniform size and arrangement," especially on fabric, 1851 (polka-spot and polka-dotted are used in 1849), when they were in fashion, from polka (n.) + dot (n.). Named for the dance, for no reason except its popularity, which led to many contemporary products and fashions taking the name (polka hat, polka-jacket, etc.). They had a revival in fashion c. 1873. Related: Polka-dots.
A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Weve used all 26 valence electrons. Bond angle of a NI3 molecule. Get the detailed answer. Are molecular or coval.
"point or minute spot on a surface," Old English dott, once, "speck, head of a boil," perhaps related to Norwegian dot "lump, small knot," Dutch dot "knot, small bunch, wisp," Old High German tutta "nipple;" a word of uncertain etymology. Known from a single source c. 1000; the word reappeared with modern meaning "mark" c. 1530; not common until 18c. Perhaps this is a different word imitative of "the mark of a mere touch with the pen" (Wedgwood). In music, the meaning "point indicating a note is to be lengthened by half" is by 1806. Morse telegraph sense is from 1838. On the dot "punctual" is 1909, in reference to a clock dial face. Dot-matrix in printing and screen display is attested by 1975.
This video is a quick crash course on how to draw Lewis Dot Structures with a given equation! To learn more about us, click this link: https://creativemindsot.carrd.co/ Sign up for free to create engaging, inspiring, and converting videos with Powtoon.
prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv
Ozone has sp2 hybridization means that it should have a trigonal planar shape. The arsenate ion is AsO3− 4. Draw the Lewis structure for ClBr3, identify if its polar or non-polar, the approximate bond angles, and its molecular geometry. ((On the homework feedback, it said it is a valid structure but not Include any nonzero formal charges and lone pair electrons in the structure.
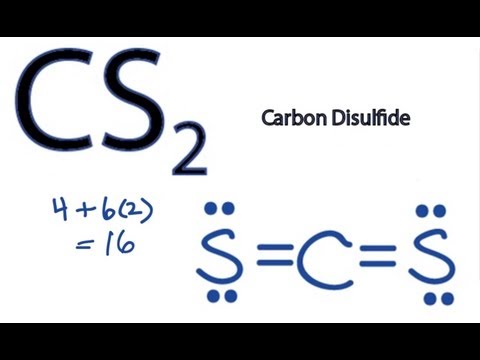
Answer: CS2 Lewis structure(carbon disulfide electron dot structure) is that type of diagram where we show the total 16 valence electrons of CS2 ...Nov 6, 2019 · Uploaded by Chemistry 360
The lewis dot structure for CS2 also predicts many of the properties of the molecule. Which of the following are possible Lewis structures for C 2H6OA. As a result there can be more induced dipoles which increases the solubility of CS2. Find the number of valence electrons for each of the atoms in the moleculeThe number of valence electrons is ...
So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. OF2 formula becomes AX2N2. According to the VSEPR chart, the molecule which has the AX2N2 formula their molecular shape is bent and electron geometry is tetrahedral.
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
A step by step explanation of how to draw the c2h2 lewis dot structure ethyne or acetylenefor the c2h2 structure use the periodic table to find the total. commonly called acetylene has o 2 single bonds 1 triple bond and 1 lone pair. C2 molecular orbital diagram. as you can see in the diagram the two 2ppi orbitals lets say 2ppix and 2ppiy are ...
A Step-by-Step Tutorial ... We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other ...Aug 11, 2013 · Uploaded by Wayne Breslyn
Draw the Lewis dot structure for. A step-by-step explanation of how to draw the CS2 Lewis Dot Structure Carbon disulfideFor the CS2 structure use the periodic table to find the total numbe. Aluminum 13 3s2 p1 Al 3. I am looking at drawing the lewis structure for the S2- ion. The Lewis structure of S2- is represented by the capital letter S that ...



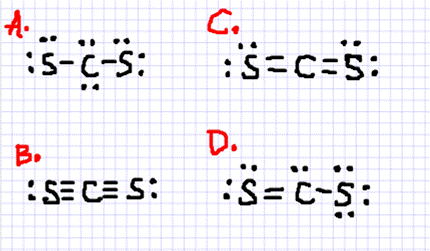







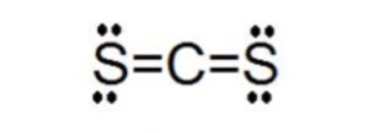
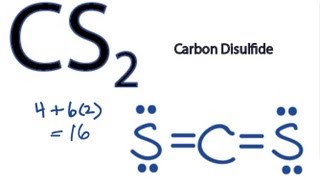








0 Response to "37 lewis dot diagram for cs2"
Post a Comment