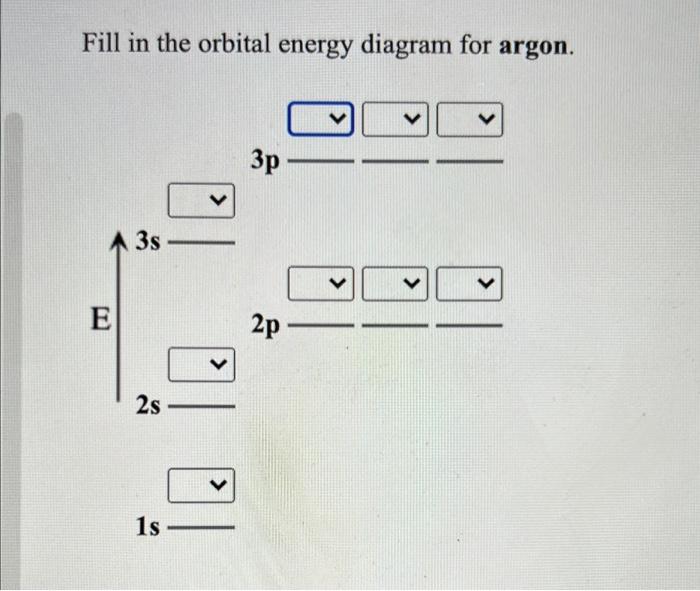
41 orbital diagram of argon
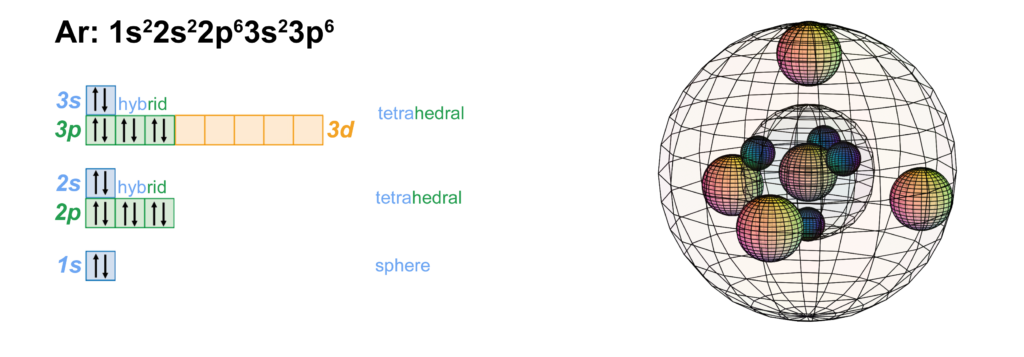
Below is the electronic diagram of the Argon atom. Orbital diagram of the Argon atom. Distribution of electrons over energy levels in the Ar atom Argon provides an inert atmosphere in which welded metals will not oxidise. Appearance. Argon is a colourless, odourless gas that is totally inert to other substances. Uses. Argon is often used when an inert atmosphere is needed. It is used in this way for the production of titanium and other reactive elements.
Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!

Orbital diagram of argon
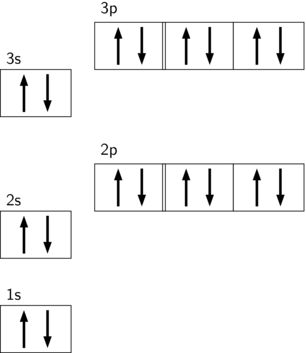
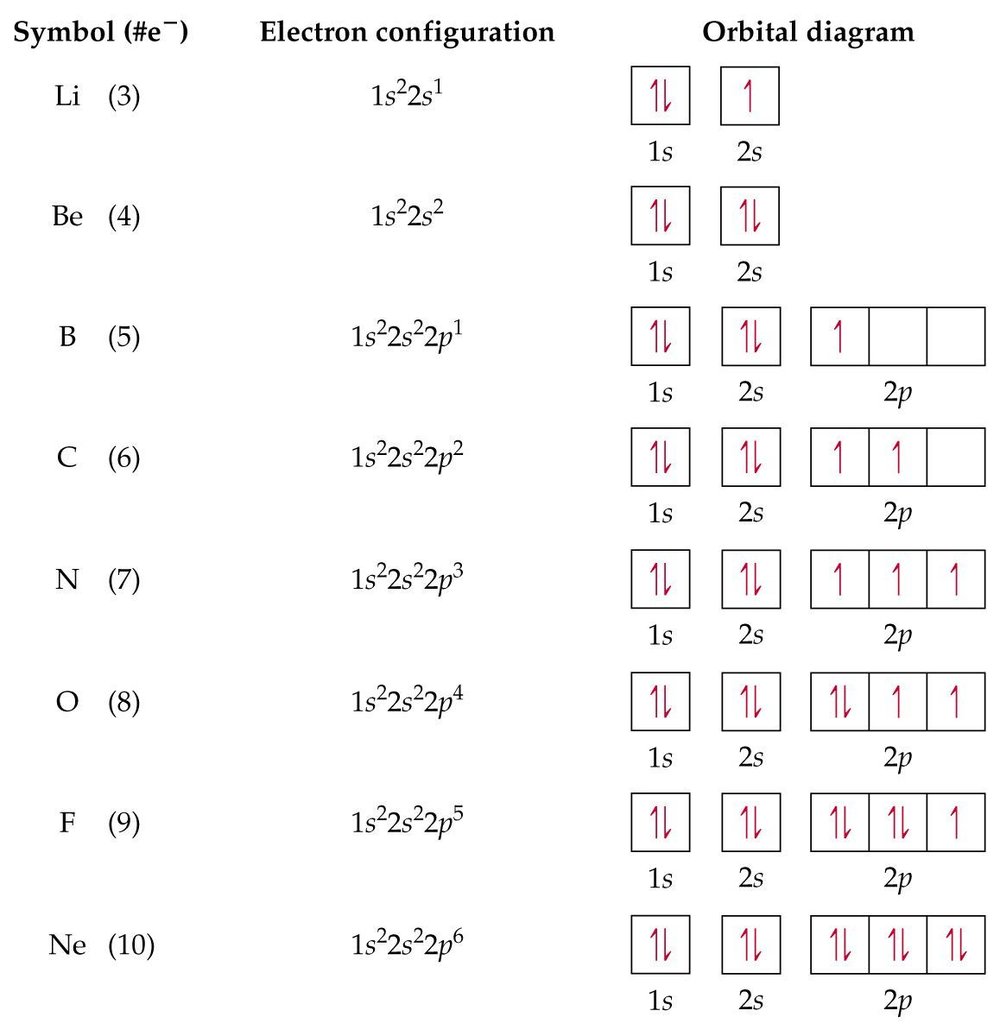
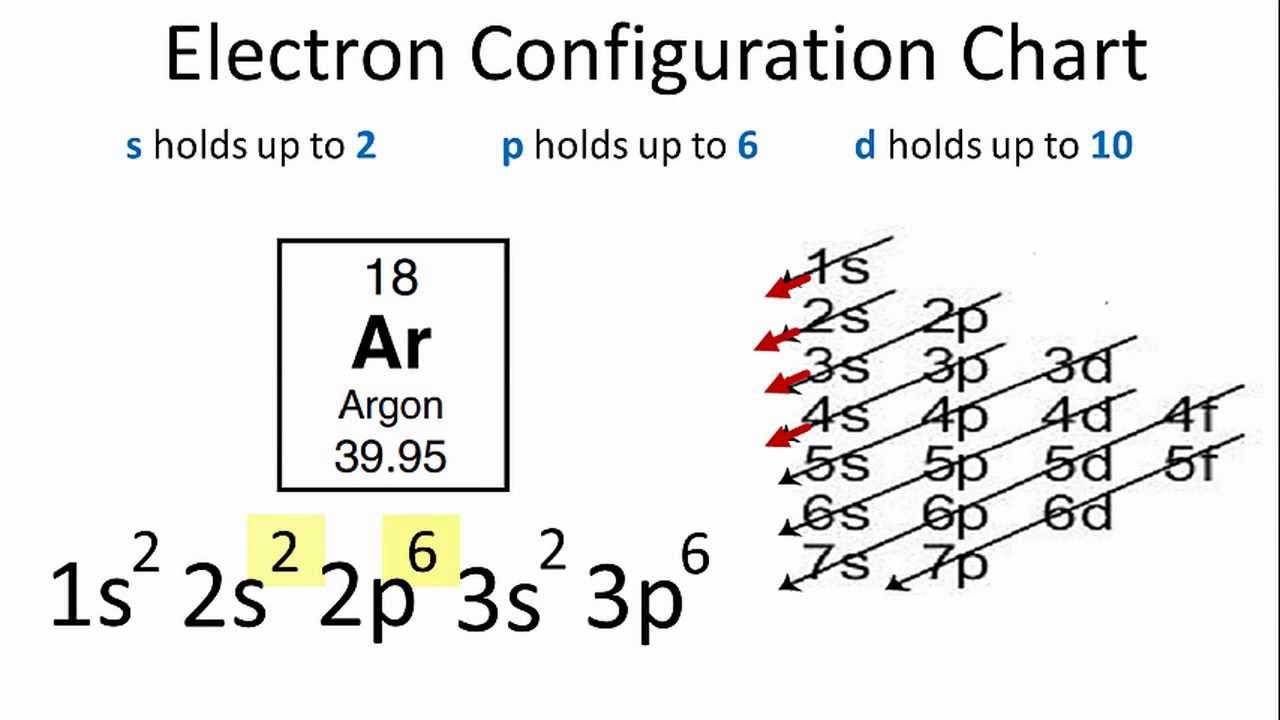
In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. 23 Feb 2012 — Convert from orbital representation diagrams to electron configuration codes. ... Potassium has the same electron configuration as argon ... Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
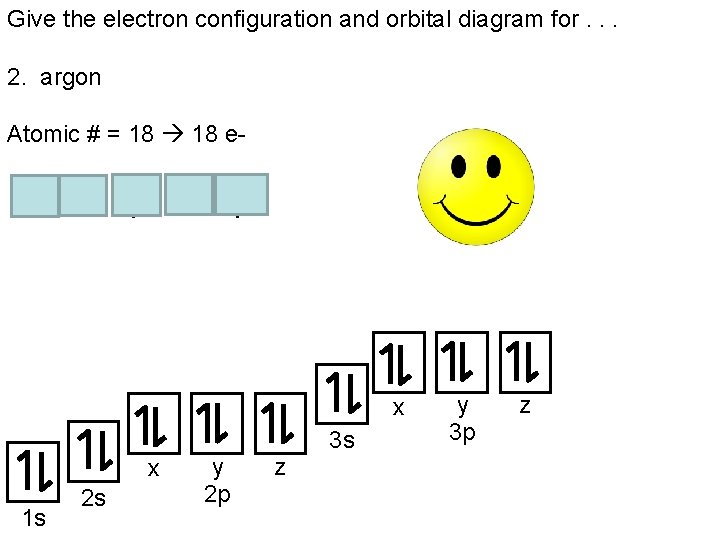
Orbital diagram of argon. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The sequence of orbitals for electron configuration can be seen from this diagram. The maximum number of electrons that can be filled in each orbital are: s = 2.1 answer · Top answer: Hey there! We have to draw the electron configuration for a neutron atom of argon.The electron configuration of an atom or molecule is the distribution ... 24 Jan 2021 — Argon's electron configuration for the first two electrons goes in the 1s orbital. 1s only hold two electrons and the next 2 electrons for Argon ... Lewis Dot Diagram of Argon (Ar). Description: Colorless, odorless, tasteless noble gas. It is the third most abundant element in the earth's atmosphere and ...
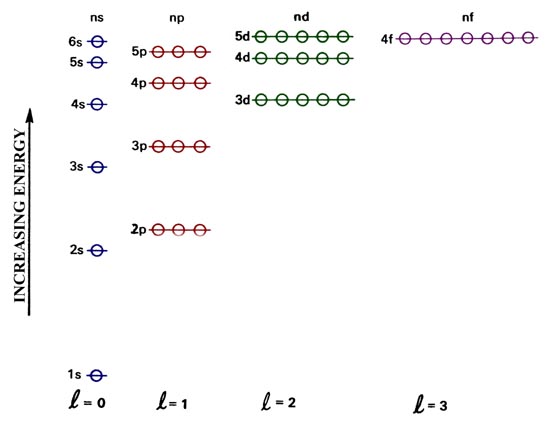
The orbital diagrams below are of argon, nitrogen, and electron. image via galleryhip.com. image via chemistryland.com. image via chemwiki.ucdavis.edu. Unlike an s orbital, a p orbital points in a particular direction – the one drawn points up and down the page. At any one energy level it is possible to have three absolutely equivalent p ... Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of ... This video shows how to create an orbital diagram of an atom from its electronic configuration Each box represents an orbital. Each orbital can hold up to two electrons. Ar. Question: Part A - Write the electron configuration for the Argon atom. Ch6: Photoelectron Spectroscopy. Seg1 Orbitals and Multi-Electron Atoms 114-OL- Course The electron energy-level diagram below shows the relative energies of orbitals for any multi- electron atom.
The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices. 23 Feb 2012 — Convert from orbital representation diagrams to electron configuration codes. ... Potassium has the same electron configuration as argon ... In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.

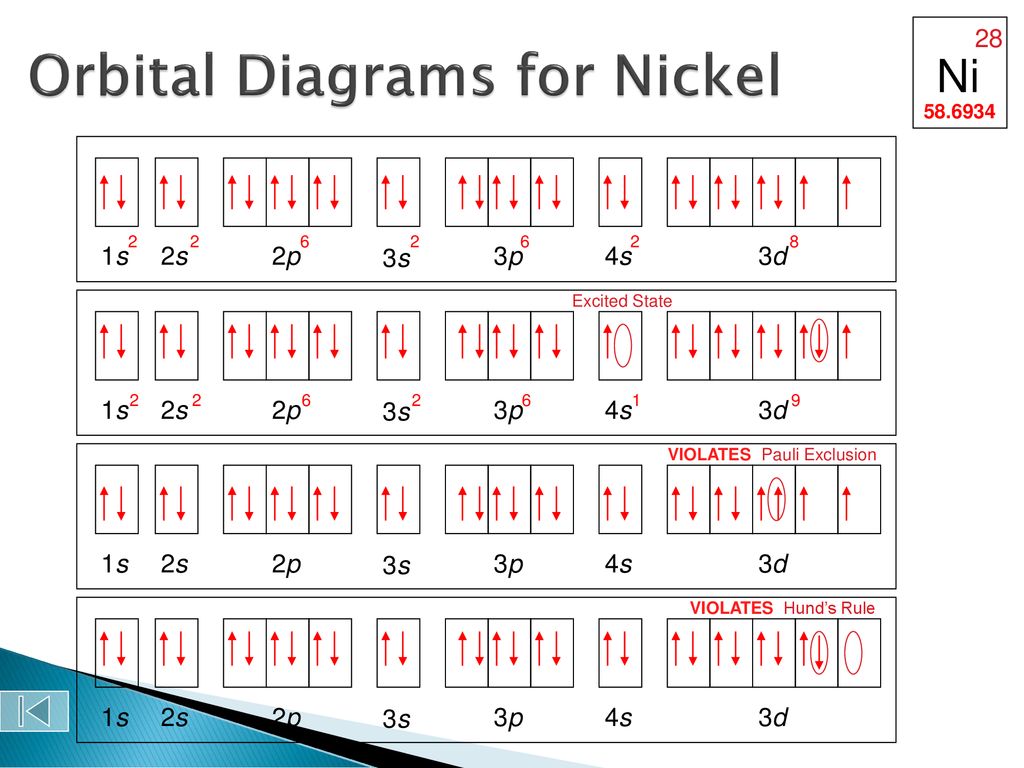
Solved Write The Complete Orbital Diagram For Each Of The Following Elements Using Boxes To Represent Orbitals And Arrows To Represent Electrons Begin Array L Text A Aluminum Z 13 Text B Phosphorus

Write Electronic Configuration Of Argon Z 18 Using Orbital Notation And Orbital Diagram Method Brainly In

Orbital Diagram Ar What Ground State Atom Has An Electron Configuration Described By The Following Orbital Diagram

Write Electronic Configuration 18 Ar And 19 K Using Orbital Notaton And Orbital Diagram Method Brainly In






















0 Response to "41 orbital diagram of argon"
Post a Comment