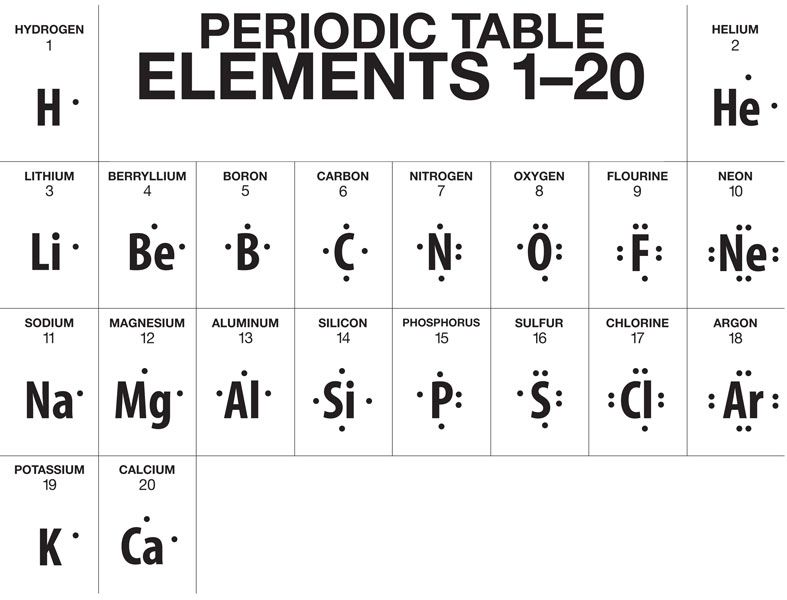
40 lewis dot diagram for phosphorus
Q. Does this lewis dot structure show the octet rule? answer choices . Yes. No. Tags: Question 11 . SURVEY . 30 seconds . Q. Is this the correct Lewis Dot Structure for phosphorus? answer choices . Yes . No. Tags: Question 12 . SURVEY . 30 seconds . Q. Is this the correct Lewis Dot Structure for Boron? answer choices . Yes. No, missing ... Phosphorus Draw the Lewis dot structure for phosphorus. Include all lone pairs of electrons; Question: Phosphorus Draw the Lewis dot structure for phosphorus. Include all lone pairs of electrons. This problem has been solved! See the answer See the answer See the answer done loading.
Iodine dichloride (ICl2-) lewis dot structure, molecular geometry, polar or non-polar, hybridization ... As the lewis diagram is all about filling the valence electron around the atoms within a molecule, hence, find the total valence electron in ICl2- molecule. ... Sulfur, phosphorus, silicon, and chlorine, etc.

Lewis dot diagram for phosphorus
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. When it bonds with another atom, it needs to have 8 total electrons to complete the valence schematron.org: Resolved. Answer to: Draw the Lewis dot diagram for phosphorus. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You...
Lewis dot diagram for phosphorus. Jul 23, 2020 · Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the “How to Draw a Lewis Dot Structure” for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. In this article, we will discuss Phosphorous trifluoride (PF3) lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, etc. “Phosphorus trifluoride is similar to carbon monoxide in that it is a gas which strongly binds to iron in hemoglobin, preventing the blood from absorbing oxygen.” Nov 16, 2021 · CO 2 7. In addition to practice, the worksheet could be used foThe former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a … Nov 23, 2021 · H3PO4 Lewis Structure. To learn the nature of bonding inside any chemical molecule, the first and foremost step is to have a basic idea about its structure. Lewis Structure diagram gives us a simple representation of any given molecular composition with the help of electron dot methodology.
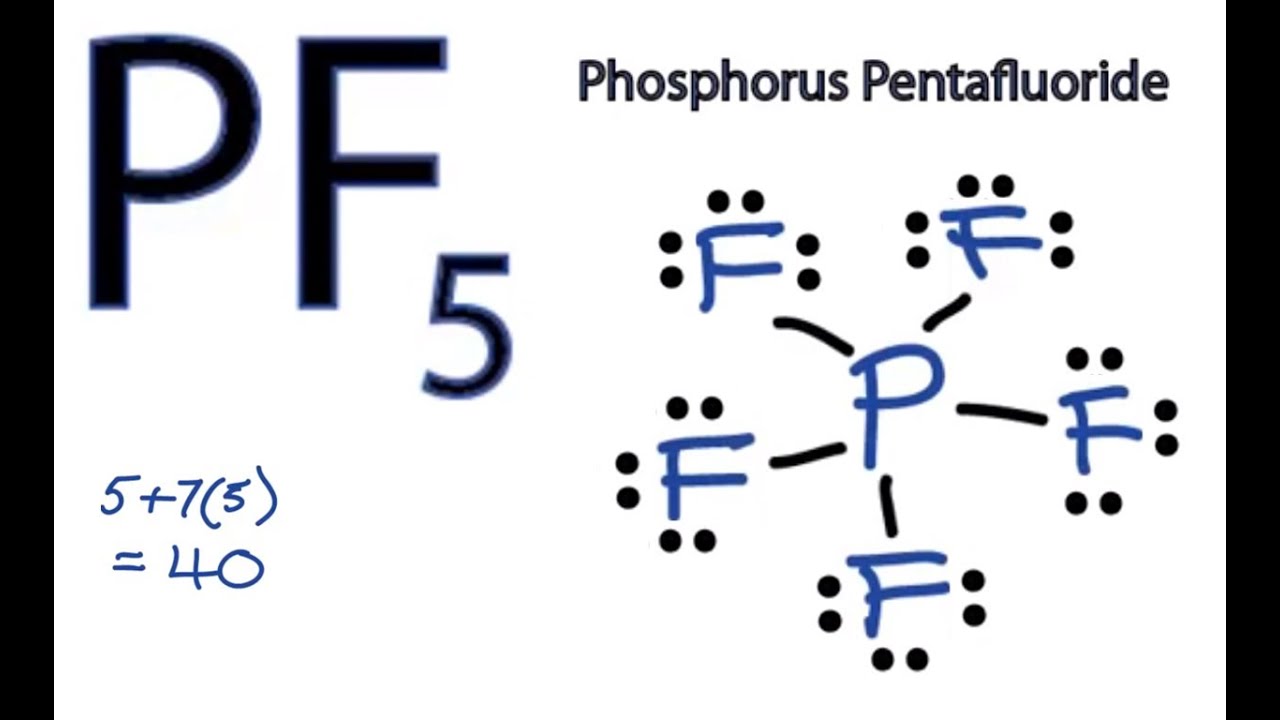
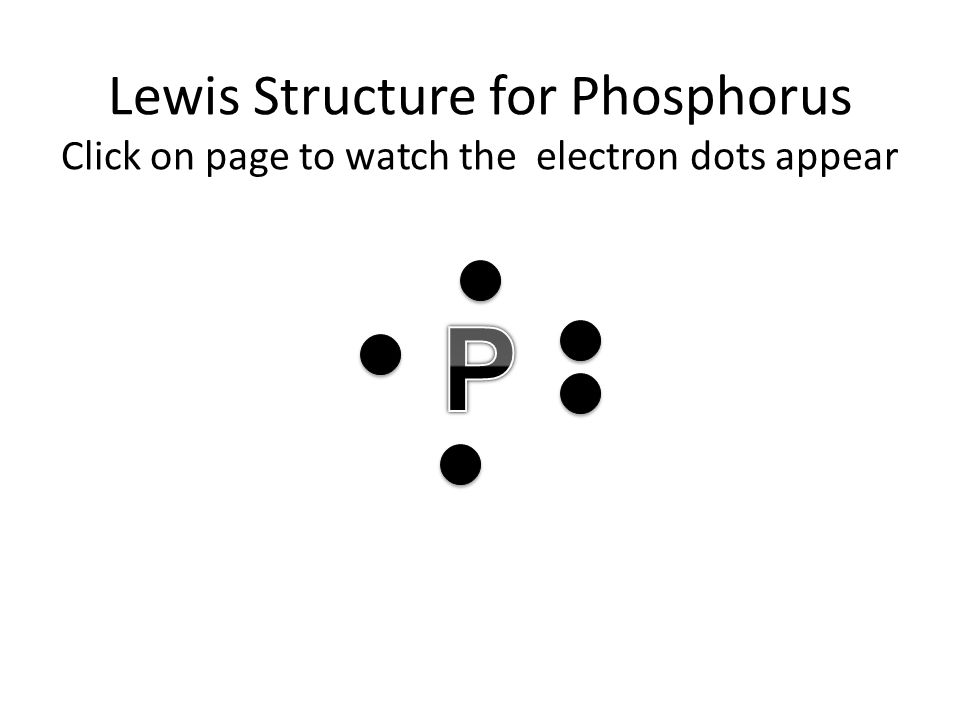
Lewis diagrams, also called electron-dot diagrams, are used to . Example: Draw the Lewis structure for phosphorus pentafluoride, PF5. The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has.U.S. Department of Transportation (DOT ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Umm lets see here, the Lewis dot diagram for phosphorus trifluoride would consist of deep depth of concentration dilemma between the two variables using the quadratic formula and postulates. screw ... How many dots would be on a Lewis diagram of phosphorus? So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
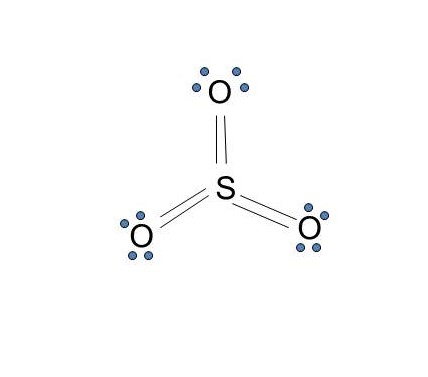
With elemental phosphorus (white phosphorus, P4) as a bonus.Check me out: http://www.chemistnate.com A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. A Lewis structure also helps to make a prediction about the geometry of a molecule. Misc hca chemistry customizable and printable lewis dot diagram worksheet 50 structure practice in 2020 (with images electron calculator free photos ppt drawing structures a tutorial on writing Lewis Structures of … P 2 O 5 (Phosphorus pentoxide) Lewis Structure. In the lewis structure of P 2 O 5, there are two elements; phosphorus and oxygen.Two phosphorus atoms are linked through an oxygen atom in the lewis structure of phosphorus pentoxide (P 2 O 5).All other oxygen atoms have made double bonds with phosphorus atoms.
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
Answer to: Draw the Lewis dot diagram for phosphorus. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You...
Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. When it bonds with another atom, it needs to have 8 total electrons to complete the valence schematron.org: Resolved.
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
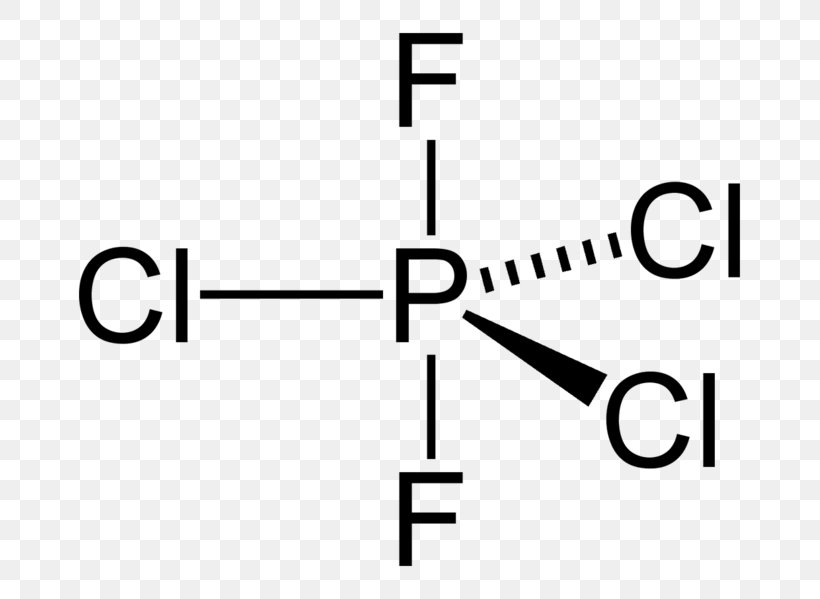
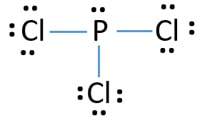
Lewis Structure Molecular Geometry Phosphorus Pentachloride Molecule Structural Formula Png 727x599px Watercolor Cartoon Flower Frame Heart

4 1 Lewis Theory Of Bonding Types Of Bonding Conditions Between Elements Low Electronegativity And Low Ionization Energy Metals High Electronegativity Ppt Download

The Electron Configuration For Phosphorous Is 1s22s22p63s23p3 What Is The Lewis Electron Dot Diagram Brainly Com

Solved Match Each Element To Its Electron Dot Diagram The Symbol X Represents The Element Refer Brainly Com

Lewis Dot Notes Lewis Dot Diagrams Illustrates The Number Of Valence Electrons Valence Electrons Number Of Electrons In Outer Shell Placed Around Ppt Download

Phosphorus Has Five Valence Electrons And Hydrogen Has One Valence Electron What Would Be The Lewis Brainly Com
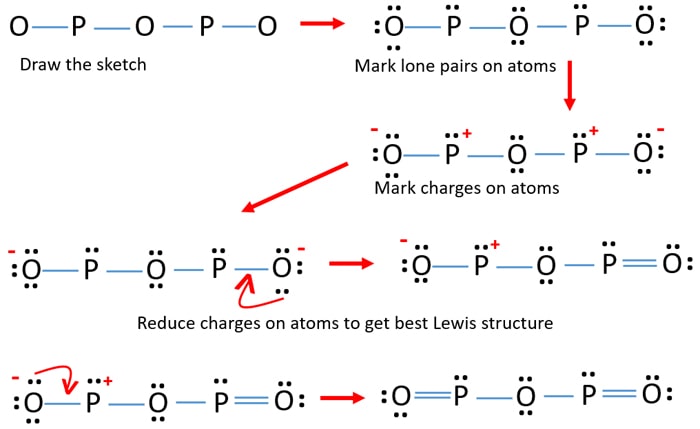





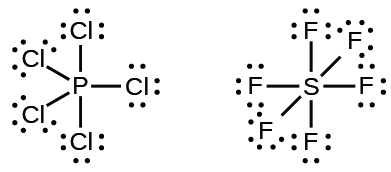












0 Response to "40 lewis dot diagram for phosphorus"
Post a Comment