40 cu-ni phase diagram
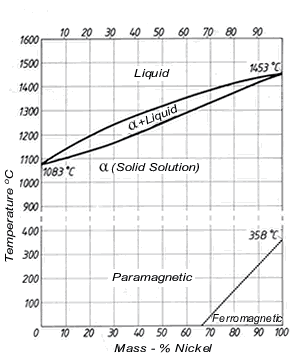
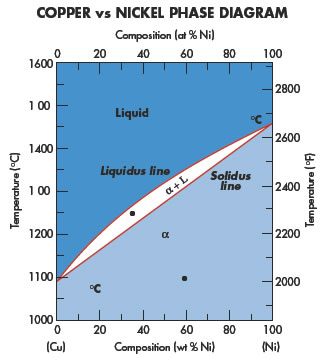
This phase is a substitutional solid solution of Cu and Ni atoms and having FCC structures. ii. At temperature below 1085°C Cu and Ni are mutually soluble in each other in solid state for all composition as both have same FCC crystal structure and nearly identical atomic radii and electronegativity. #modimechanicalengineeringtutorials, #mechanicalmagicmechanicallearningtutorials,Welcome to My YouTube Channel MODI MECHANICAL ENGINEERING TUTORIALS.This ch...
There is two types of important phase diagrams are used one is a slow process diagram, and another is a fast process diagram. Answer and Explanation: 1 Phase diagram of the Ni-Cu is
Cu-ni phase diagram
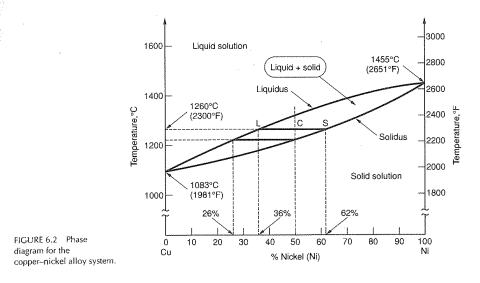
In this work, phase relations and thermal stabilities of equilibrium phases in the Cu-Ni-S system have been reviewed. The calculated phase diagram of Cu-N system has been validated. At T > 630 K, in the N-rich corner, large scatter in data has been observed and discussed in detail. Example: Cu-Ni phase diagram (only for slow cooling conditions) Liquidus line: the line connecting Ts at which liquid starts to solidify under equilibrium conditions Solidus: the temperature at which the last of the liquid phase solidifies Between liquidus and solidus: P =2. Chapter 8 9 The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or liquid or exist as two phases in equilibrium.
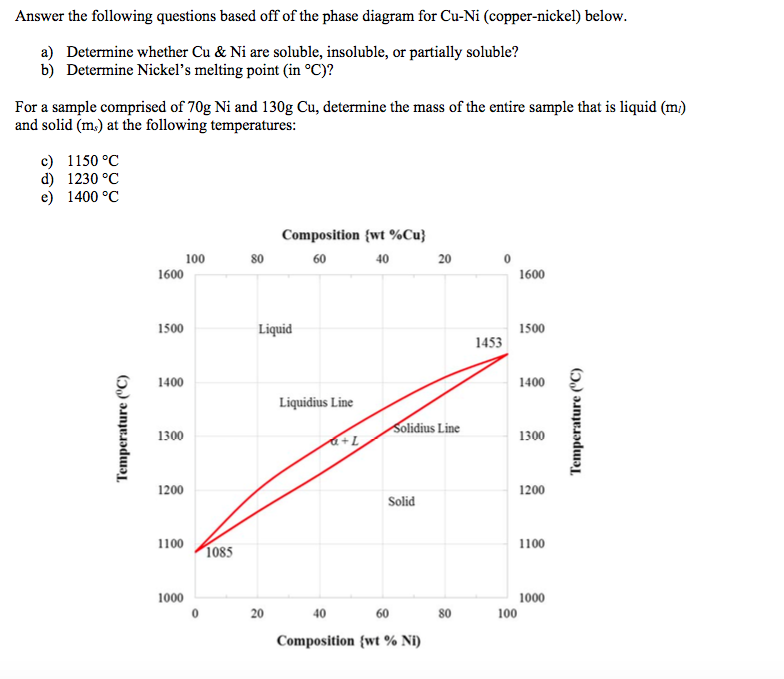
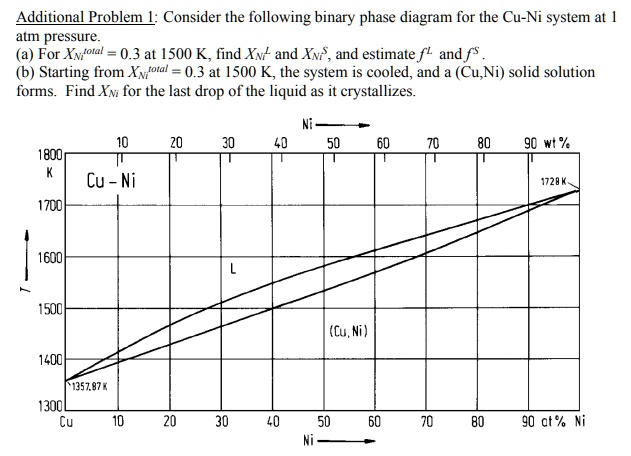
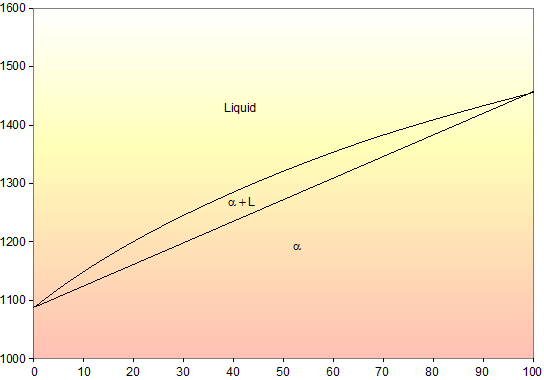
Cu-ni phase diagram. Cu-Ni phase diagram is given below. 1500 1400 L 40 1300 Q+L 1270 Temperature ("C) 37 32 50 45 1250 1200 40 a 1100 1000 Cu 20 40 60 80 Ni Weight percent nickel Answer the questions according to the diagram for 50% CU-40% Ni alloy. a. How many phase (s) present at 1270°C. Answer: b. What is the liquid phase composition at 1270°C. Answer: wt%Ni c. Cu-Ni phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins. Part of the phase diagram of the Cu-Ni-Mn system from 0 to 20% Ni and from 30 to 50% Mn is refined with the help of a theoretical analysis and based on the experimental data. Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C.
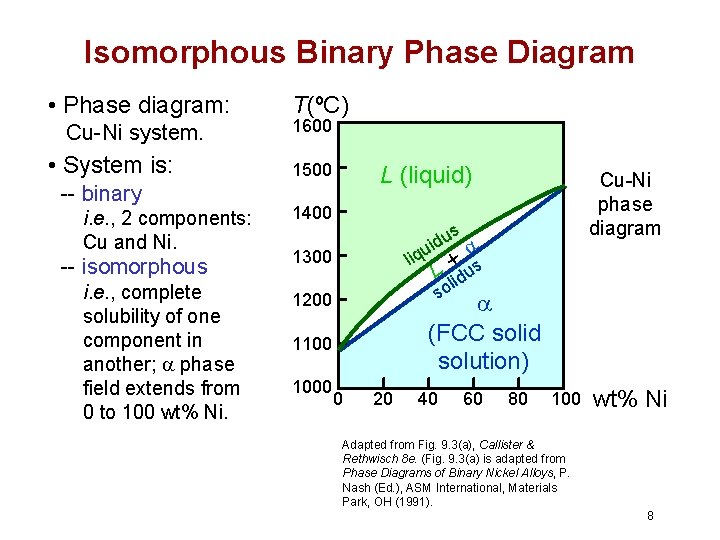
Move the mouse over the phase diagram to view temperature and composition at any point. View micrographs for the Cu-Ni system. List all systems with phase diagrams The Cu-Ni-Ti system was reviewed by [1990Gup] with literature available until 1988. Subsequently, several new results were published and some Russian literature not available earlier could be obtained. This update on the Cu-Ni-Ti system is based on the available published literature up to 1997. This video explains binary phase diagrams, specifically the Cu-Ni System. For further studies: visit https://www.doitpoms.ac.uk/tlplib/phase-diagrams/printal... • Phase diagram: Cu-Ni system. •System is:--binary i.e., 2 components: Cu and Ni.--isomorphous i.e., complete solubility of one component in another; aphase field extends from 0 to 100 wt% Ni. • Consider Co = 35 wt%Ni. Equilibrium Cooling in a Cu-Ni Binary System Chapter 9 - 12 • C a changes as we solidify. • Cu-Ni case: • Fast rate ...
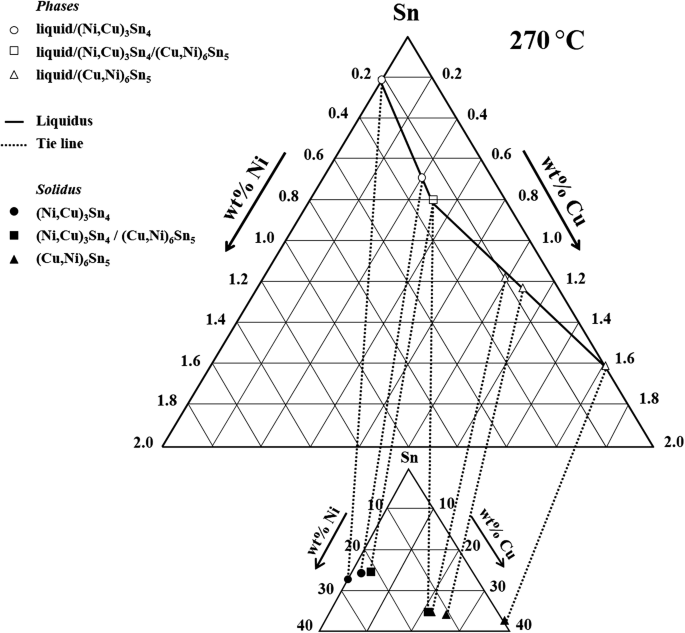
The Cu-Ni and binary phase diagram (Figure 10.3) is the simplest type of binary phase diagrams for two metals. Figure 10.3 shows that Cu and Ni are mutually soluble at room temperature throughout the entire range of compositions. Remember from Chapter 5that we discussed the HumeRothery - 7.3. Al-Cu-Ni PHASE DIAGRAM This phase diagram is helpful in the analysis of 2618-type heat-resistant alloys and 339.0-type piston alloys that contain nickel, copper, and other alloying components (Tables 7.1 and 7.2). The ternary Al7Cu4Ni phase forms in the aluminum corner of the Al-Cu-Ni system. The Sn-Cu-Ni has become increasingly significant as it is used in several solder alloys and more generally both Cu and Ni are common substrates that interact with Sn-based solders in microelectronic applications. However, there is currently an insufficient understanding of the phase equilibria and the associated phase diagrams of many Sn alloys. Answer to: For the Copper (Cu) Nickel (Ni) binary phase diagram shown. determine the following for an alloy with C0=W0=53wt% Ni 1) What is the...
Cu-Ni-Y Isothermal Section of Ternary Phase Diagram Cite this page. Citation Phase diagram available to subscribers; If you are having trouble in accessing ...
pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. An example of a phase diagram for a ternary alloy is shown for a fixed T and P below. Phase diagrams for binary systems ternary phase diagram of Ni-Cr-Fe
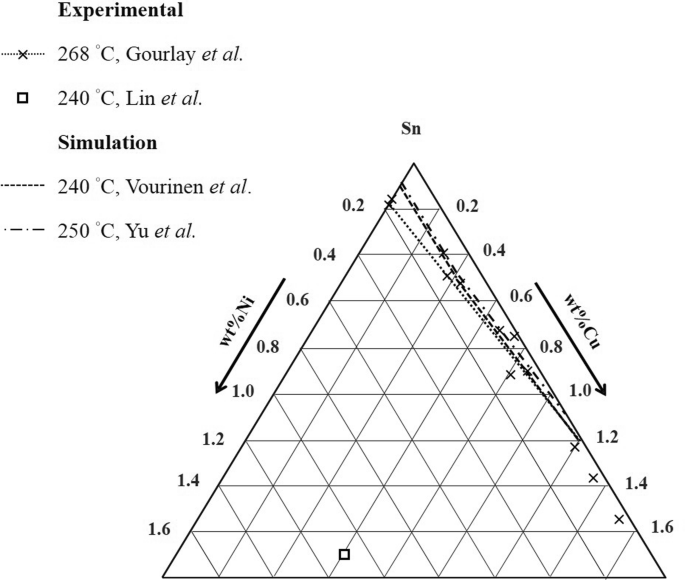
Experimental Determination Of The Sn Cu Ni Phase Diagram For Pb Free Solder Applications Springerlink
The Al 7 Cu 4 Ni 1 phase (i.e. τ), with a small homogeneity range, is the only ternary phase in the Al-Cu-Ni system [10,35,36]. The Al 7 Cu 4 Ni 1 phase has a CsCl-type base structure, where along the c axis 8 Al atoms alternates with 6 heavy atoms (i.e. Cu and Ni) and 2 vacant sites .
0. Phase Diagram 1. Overview 2. Heating & Pouring 3. Solidification and Cooling 2 0. Alloys and Phase Diagram • Pure Metals • Alloys - Solid solutions • Substitutional Solid Solution (Zn/Cn and Cu/Ni) - Atomic radii is similar - Lattice type is the same • Interstitial Solid Solution - Smaller atoms are interstitially located ...
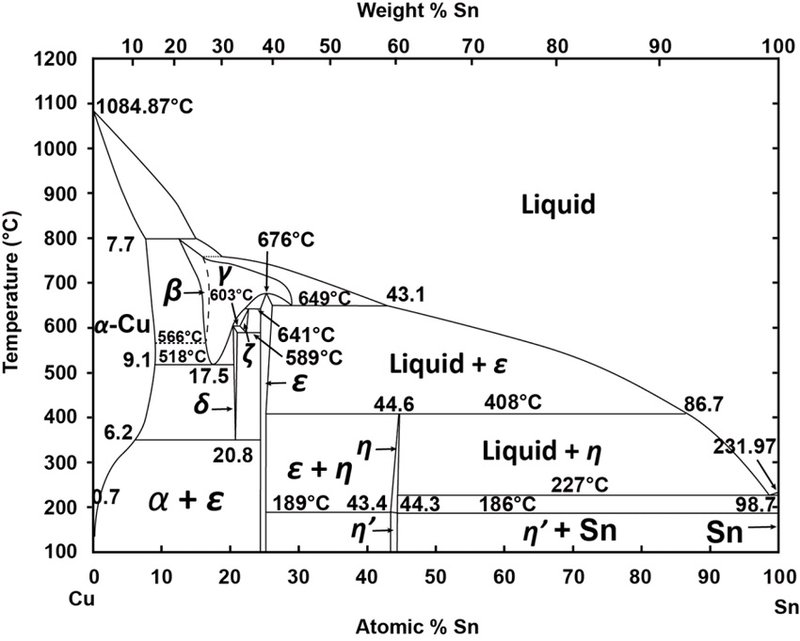
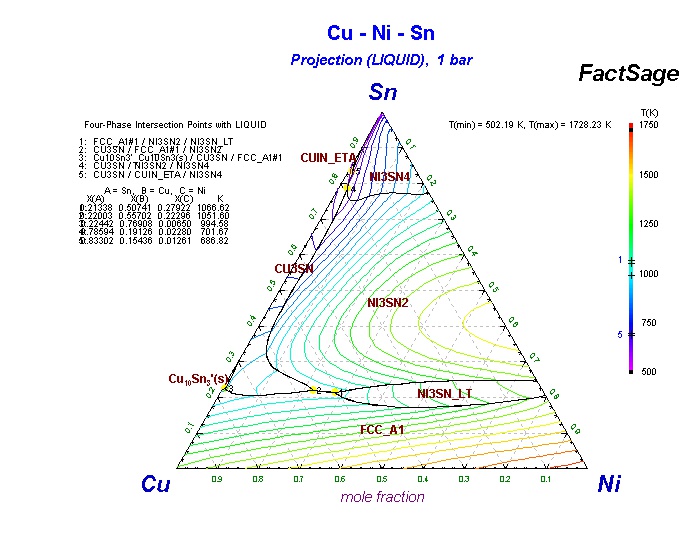
Cu-Ni-Sn description. 2. Phases and models Fig. 1 shows calculated phase diagrams for binaries Cu-Ni [21]andCu-Sn[35]. They agree well with the experimental phase equilibrium data as presented in these studies. The Ni-Sn system was recently assessed by Ghosh [32], who treated the liquidandfccphases with the
The density of copper (8.93 kg/dm 3 at 20 °C) varies only slightly with increasing nickel content (density of nickel at 20 °C = 8.9 kg/dm 3) and is 8.9 kg/dm3 for all Cu-Ni alloys specified in DIN 17 664. This aspect can also be seen in Table 7 with the physical properties of the Cu-Ni resistance alloys to DIN 17 471.
Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C. )-)-
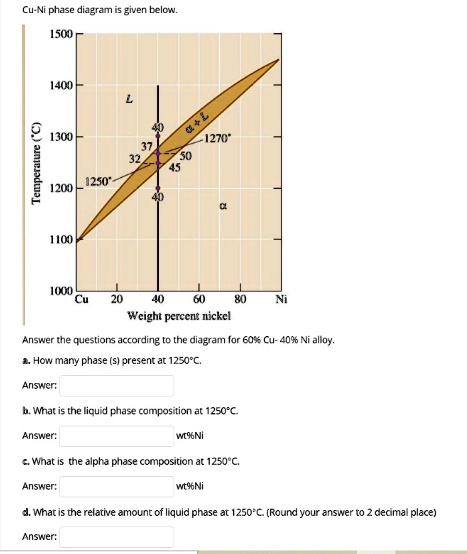
Solved Cu Ni Phase Diagram I5 Given Below I500 1400 2 1300 L 1200 0250 1270 Tm Iuu Wcight Percent Nickcl Answer Ne Questions According The Diagram For A 0 Cu 4090 Allo Hor
The Cu-Ni nanoalloy phase diagram respecting the nanoparticle size as an extra variable was calculated by the CALPHAD method. The samples of the Cu-Ni nanoalloys were prepared by the solvothermal synthesis from metal precursors. The samples were characterized by means of dynamic light scattering (DLS), infrared spectroscopy (IR ...
We have examined isomorphous phase diagrams, and used the example of the Cu-Ni phase diagram. In this module we will examine eutectic phase diagrams. A eutectic system has two components, and they have limited solubility. Upon cooling, the liquid will transform into two mixed solid phases. We will use the Pb-Sn phase diagram as an example.
In this lecture unary phase diagram of water has been explained. Which contains the explanation of three single phase region, Phase Boundary lines separating...
The binary phase diagram is used for system of two components and its classifications depend on number of phases as; isomorphous contain system with two phases such as Cu-Ni system.

For The Copper Cu Nickel Ni Binary Phase Diagram Shown Determine The Following For An Alloy With C0 W0 53wt Ni 1 What Is The Liquidus And Solidus Temperature At This Composition Co W0 53wt
Examples Cu-Ni phase diagram Temperature- Composition Binary isomorphous diagram CO2 phase diagram Pressure-Temperature Unary diagram 7. A thermodynamic law which governs the conditions for phase equilibrium. Useful in interpreting Phase Diagrams. P+F=C+N P is the number of phases present F is termed the number of degrees of freedom C in ...
(a) Cu-Ni Let us begin with the simplest of all metallic binary systems; one in which there is complete solubility (mixing and complete miscibility of atoms) in both the liquid and solid states. Alloys of copper (Cu) and nickel (Ni) are an example of such a system. The phase diagram for Cu-Ni is shown in Figure 1203.01.02, where the diagram
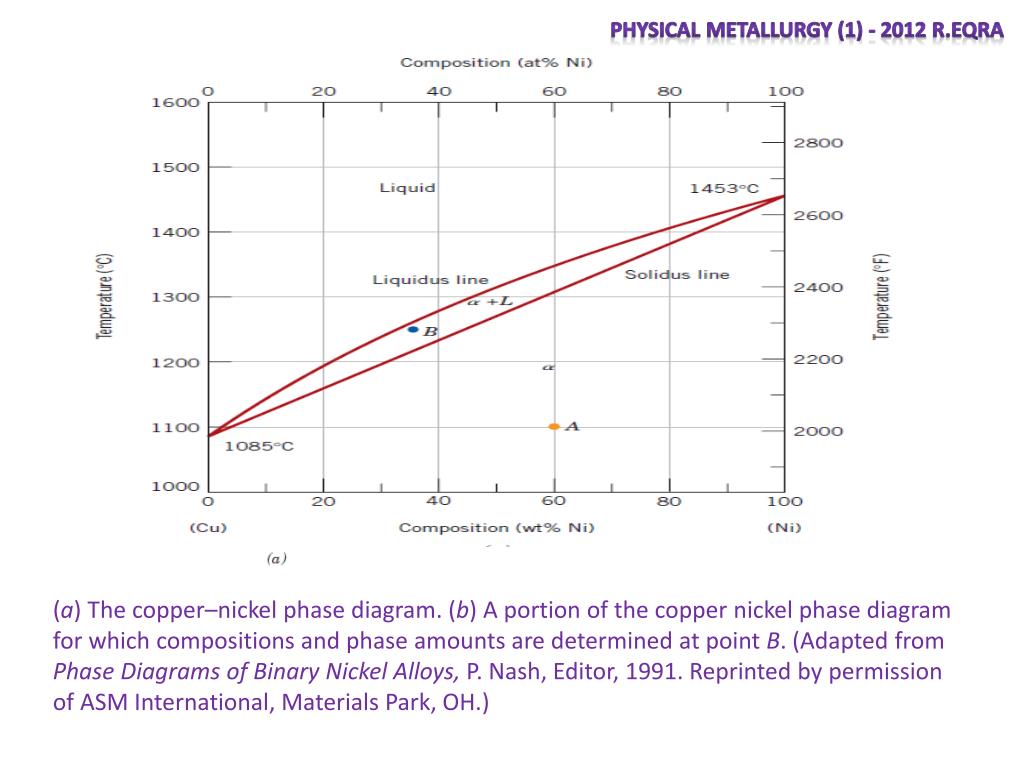
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash
TDmeph IRSN Mephista nuclear database (0) TDnucl IRSN Nuclea nuclear database (0) SpMCBN Spencer Group M-C-N-B-Si alloys (837)
The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or liquid or exist as two phases in equilibrium.
Example: Cu-Ni phase diagram (only for slow cooling conditions) Liquidus line: the line connecting Ts at which liquid starts to solidify under equilibrium conditions Solidus: the temperature at which the last of the liquid phase solidifies Between liquidus and solidus: P =2. Chapter 8 9
In this work, phase relations and thermal stabilities of equilibrium phases in the Cu-Ni-S system have been reviewed. The calculated phase diagram of Cu-N system has been validated. At T > 630 K, in the N-rich corner, large scatter in data has been observed and discussed in detail.
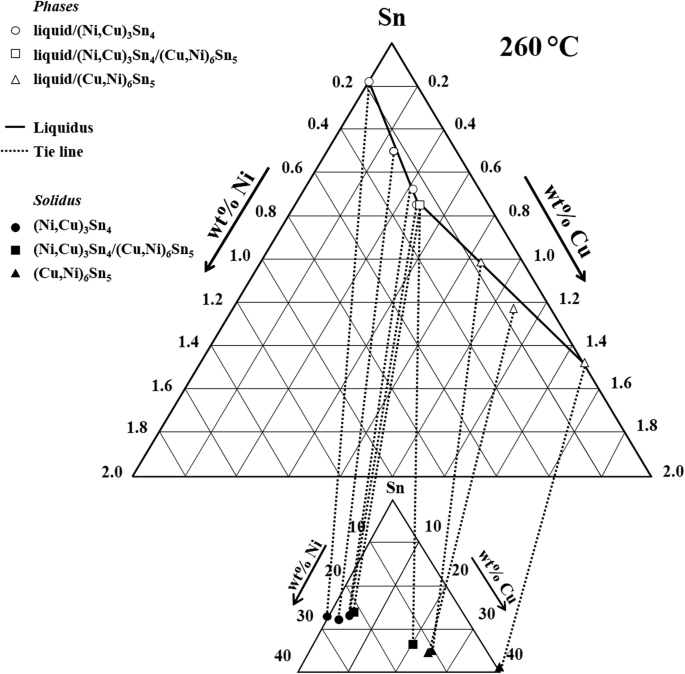
Experimental Determination Of The Sn Cu Ni Phase Diagram For Pb Free Solder Applications Springerlink

In The Binary Phase Diagram For The Cu Ni System Below Draw The Microstructures For Each Point Homeworklib
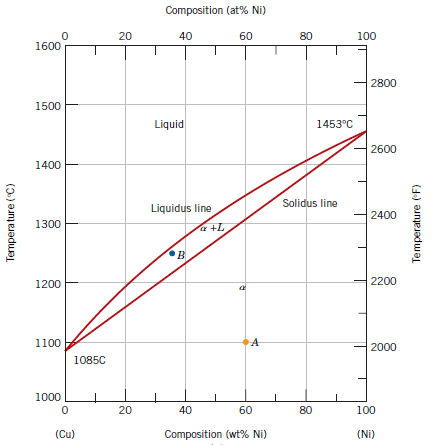
Phase Diagram Numerical Problem Part Ii Material Science Numerical Problem Material Science Questions And Answers 2020














0 Response to "40 cu-ni phase diagram"
Post a Comment