38 co molecular orbital diagram
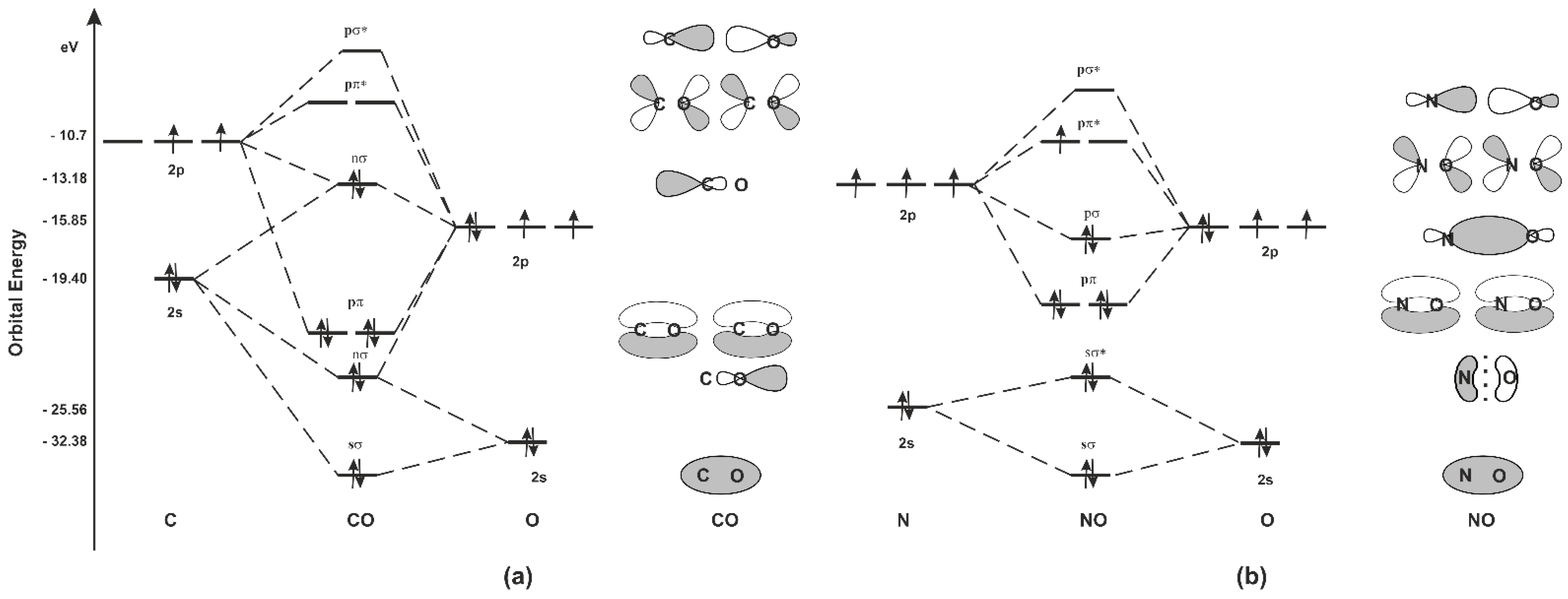
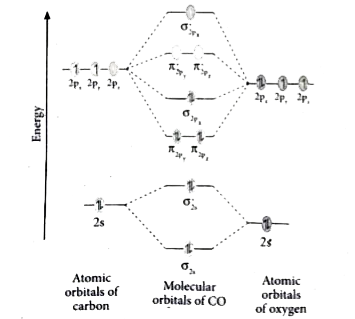
Free PDF download of Important Questions for CBSE Class 11 Chemistry Chapter 4 - Chemical Bonding and Molecular Structure prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books. Register online for Chemistry tuition on … The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence ...
January 28, 2017 - Molecular orbital diagram of H2+ (Hydrogen molecule ion) : ... O2 molecule is paramagnetic because two unpaired electrons are present. One unpaired in π*2py1 and one in π*2pz1. ... This is superoxide ion. It has 8+8+1=17 electrons. The M.O configuration is O2– is

Co molecular orbital diagram
Measured CO bond length is 1.128 Å, & bond length of CO+ is 1.115 Å. · * The Bond Order in CO+ is 3.5 . · The highest occupied molecular orbital (or HOMO) is the ...4 answers · 29 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ... 18.03.2018 · Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the ... November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
Co molecular orbital diagram. January 25, 2017 - According to my books the bond order of $\ce{CO+}$ is $3.5$. But shouldn't it be $2.5$? On googling this, I found the following answer that is on Stack Exchange but its only talks about the bond l... August 15, 2017 - For ul("O"_2^(-)), just take the MO diagram of "O"_2 and add one electron into the pi_(2px)^"*" antibonding molecular orbital. For ul("CO"^(+)), just take out one of the electrons from the 3sigma HOMO from the MO diagram of "CO". Both substances are paramagnetic, as they have at least one unpaired ... November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ... The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital. He (Z = 2): 1s 2. The third electron goes into the next orbital in the energy diagram, the 2s …
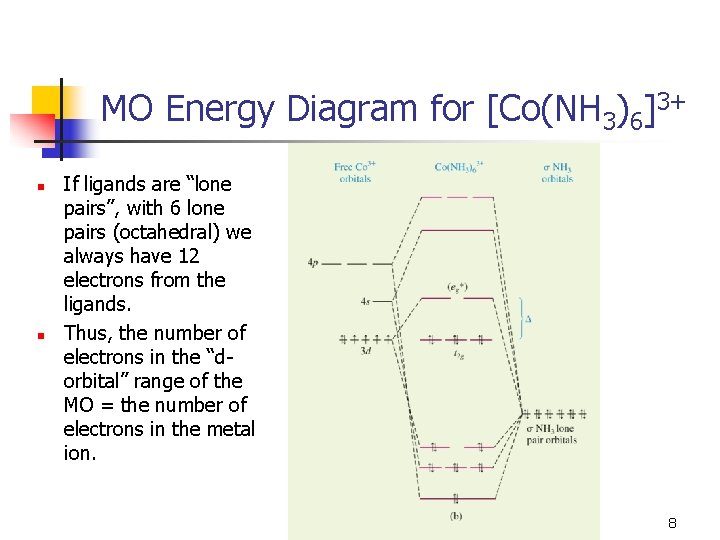
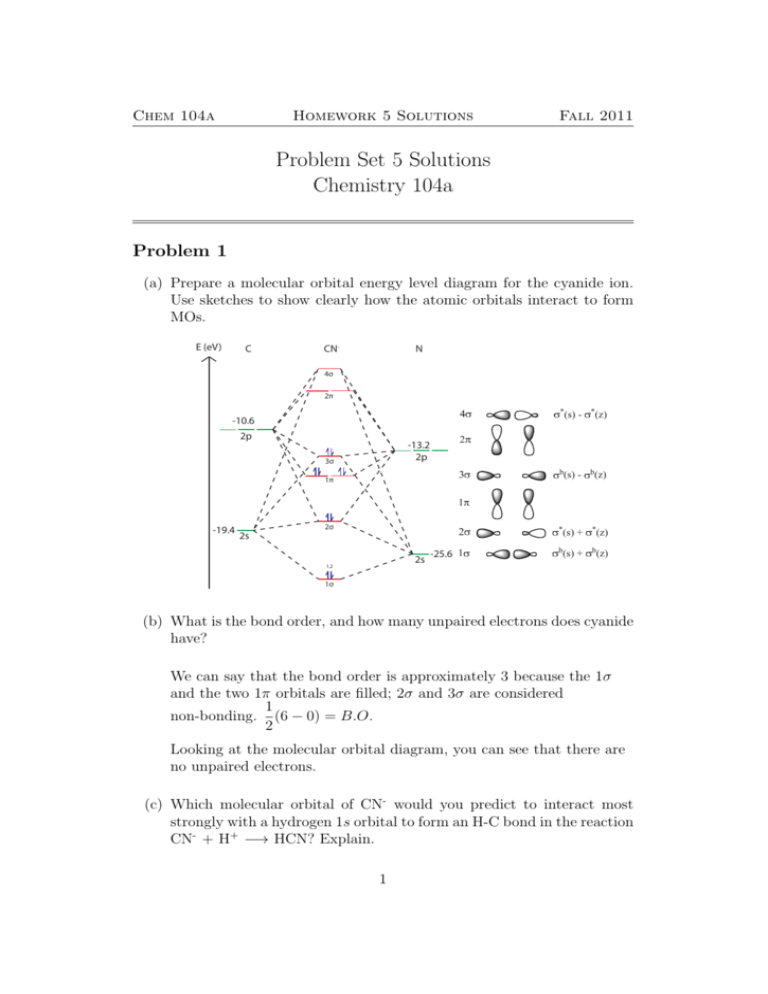
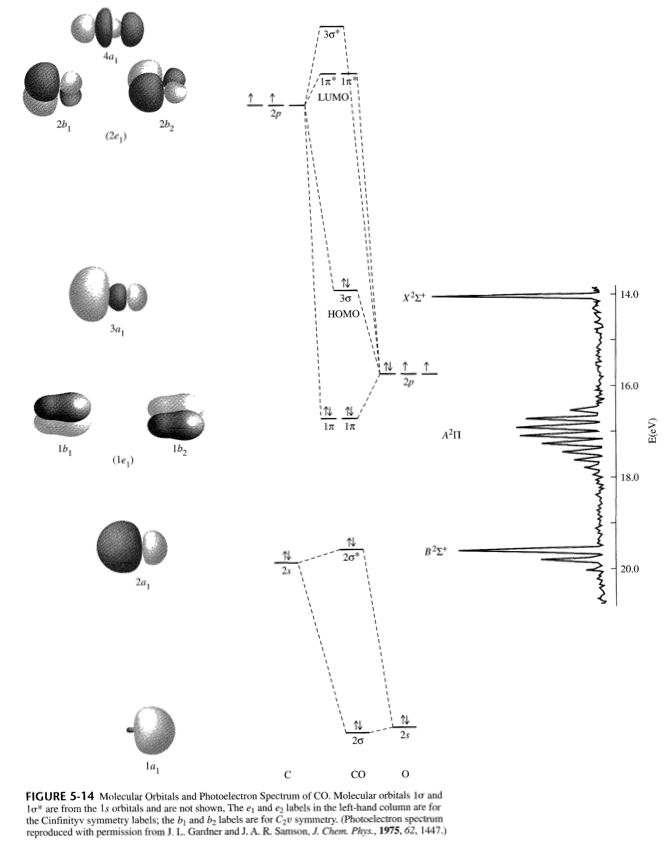
September 21, 2016 - Understandably, the key difference ... differences in energy between the molecular orbital and the atoms. But I can't explain why $\ce{CO}$ is a two-electron donor using MO theory, even though I can with Lewis? ... In the above MO diagram, the 5σ is the HOMO.... 11.08.2021 · In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward answer: use this formula: Bond order = [(Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2 . Bond order and magnetic moment of CO + is A 25 and paramagnetic moment B 35 and diamagnetic moment C 35 and paramagnetic moment D 25 and diamagnetic moment NCERT Solutions for Class 11 Chemistry Chapter 4: Chemical Bonding and Molecular Structure “Chemical Bonding and Molecular Structure” is the fourth chapter of the term – I CBSE Class 11 Chemistry Syllabus for session 2021-22. This chapter touches on several fundamental concepts in the field of Chemistry (such as hybridization and the modern theories on chemical bonding).
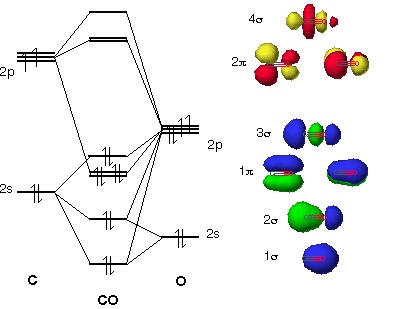
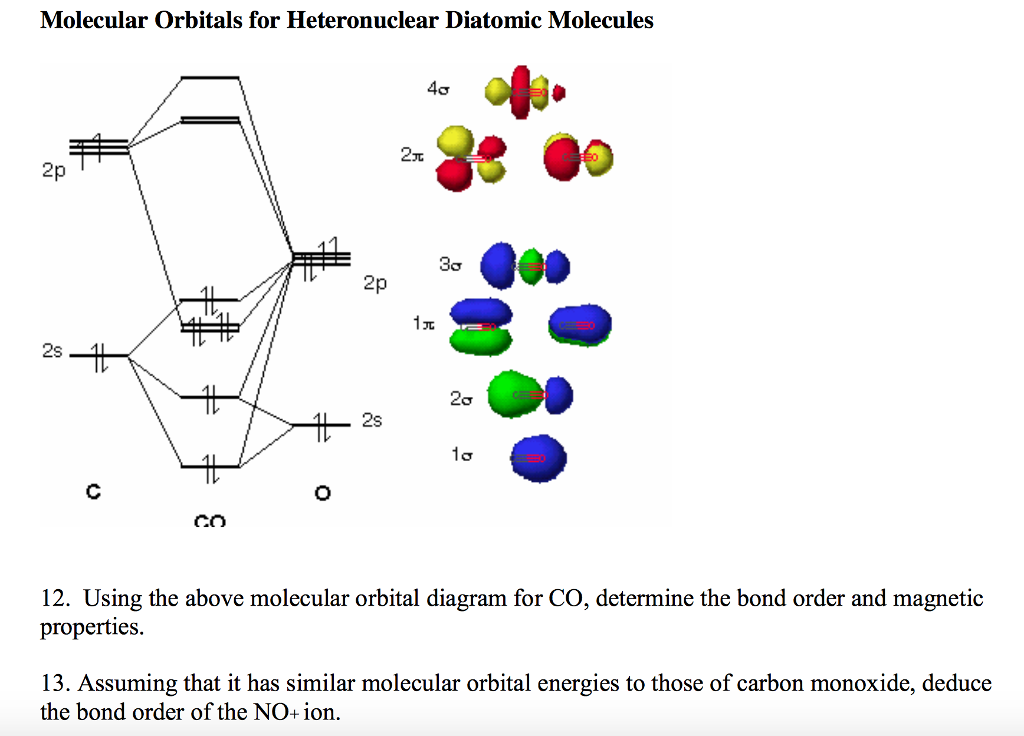
Carbon monoxide (chemical formula CO) is a colorless, odorless, tasteless, flammable gas that is slightly less dense than air.Carbon monoxide consists of one carbon atom and one oxygen atom. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl.It is a key ingredient in many processes in industrial chemistry. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that ... Theory: From H 2 to Data-Storage Alloys The COHP (or COOP) concept is most easily understood by looking at the simple band structure of a "one-dimensional" solid; the following example has been stolen from a classic introduction.Imagine a linear chain of hydrogen atoms, the one-dimensionally periodic analogue of H 2 (whose molecular-orbital scheme is known from the freshmen lecture)! Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules.
23:32In this video we are discuss about MO Diagram and Characteristics of CO Molecule MO diagrams of ...19 Oct 2019 · Uploaded by Chem Academy
February 3, 2017 - Answer (1 of 6): Measured CO bond length is 1.128 Å, & bond length of CO+ is 1.115 Å. * The Bond Order in CO+ is 3.5 . The highest occupied molecular orbital (or HOMO) is the σ *2s MO. Bond order is defined as the number of electrons in bonding MOs minus the number of electrons in antibonding ...
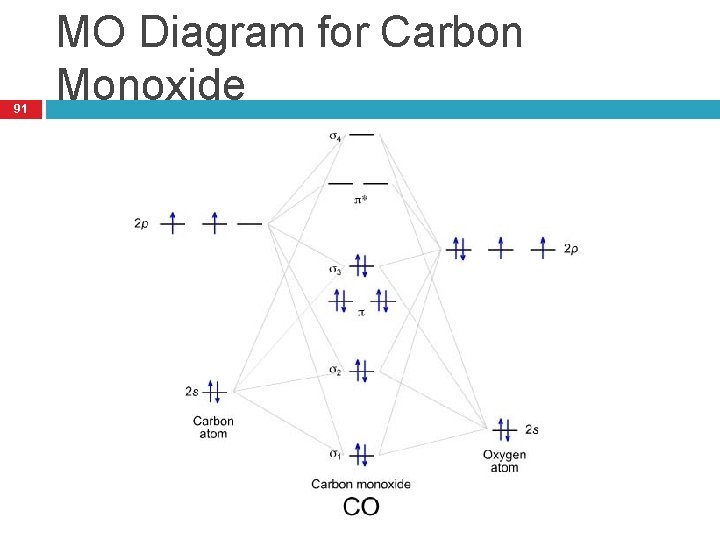
March 19, 2021 - The molecular orbital diagram for carbon monoxide (Figure \(\PageIndex{1}\)) is constructed similarly to how you would construct dicarbon or dioxygen, except that the oxygen orbitals have a lower potential energy than analogous carbon orbitals. The labeling of molecular orbitals in this diagram ...
12-12 This video describes the molecular orbital theory diagram of CO, placing emphasis on how MO theory differs for homo and heteronuclear diatomics
How bond order is calculated and why co+ has bond order 3.5? Find the answer to this question along with unlimited Chemistry questions and prepare better for JEE 2020 exam.
Download scientific diagram | Molecular orbital diagram of CO. from publication: 604-4481-2-PB | The Chemistry of Group-VIb Metal Carbonyls | | ResearchGate, the professional network for scientists.

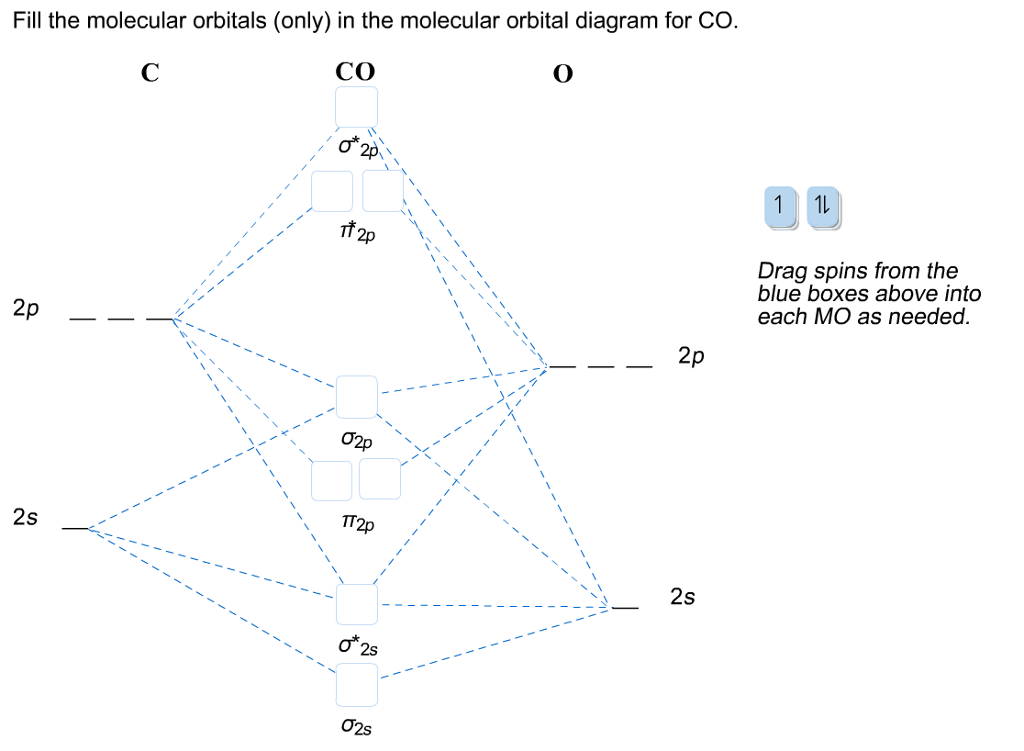
Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
Besides the shortfalls in the total contributions to MOs, the Table shows also that each NAO is not wholly accounted for. This is because the rest maps to even higher virtual MOs ... The σ orbitals (black in the Energy Level Diagram) lie symmetrically across the π nodes of the πx or πy ...
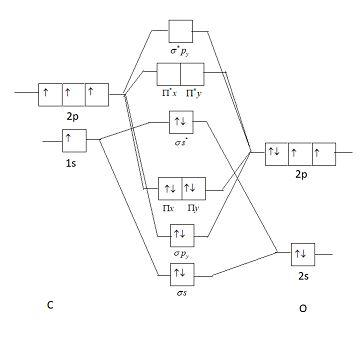
Draw MO diagram of CO and calculate its bond order.
Click to go to the main page · botslinks
Ijms Free Full Text Carbon Monoxide And Nitric Oxide As Examples Of The Youngest Class Of Transmitters Html
This is a schematic diagram of a rod cell. The stacked disks contain rhodopsin, the complex of opsin protein and 11- ... This event is best understood in terms of molecular orbitals, orbital energy, and electron excitation. You may find it helpful to review these important concepts in the introduction to the Experiment in your lab manual, or in your chemistry textbook. In the Experiment, you ...
Certain molecules such as xenon difluoride and sulfur hexafluoride have higher co-ordination numbers than would be possible due to strictly covalent bonding according to the octet rule.This is explained by the three-center four-electron bond ("3c–4e") model which interprets the molecular wavefunction in terms of non-bonding highest occupied molecular orbitals in molecular orbital theory and ...
Access 130+ million publications and connect with 20+ million researchers. Join for free and gain visibility by uploading your research.
November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
18.03.2018 · Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the ...
Measured CO bond length is 1.128 Å, & bond length of CO+ is 1.115 Å. · * The Bond Order in CO+ is 3.5 . · The highest occupied molecular orbital (or HOMO) is the ...4 answers · 29 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ...
Draw Mo Diagram Of Co And Calculate Its Bond Order Sarthaks Econnect Largest Online Education Community

1 Draw The Molecular Orbital Diagram Of Transition Metal Ion In High Spin Mn H2o 4 Oh 2 Complex Also Determine Homeworklib
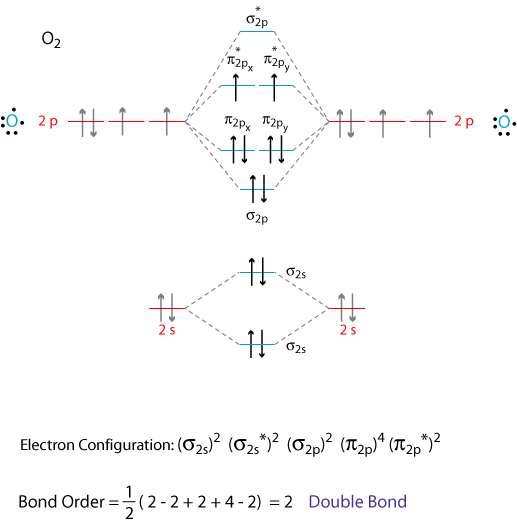
By Writing Molecular Orbital Configuration For No Co O2 Molecules Calculate The Bond Order And Also Determine Whether It Is Paramagnetic Or Diamagnetic Socratic














0 Response to "38 co molecular orbital diagram"
Post a Comment