37 molecular orbital diagram practice worksheet
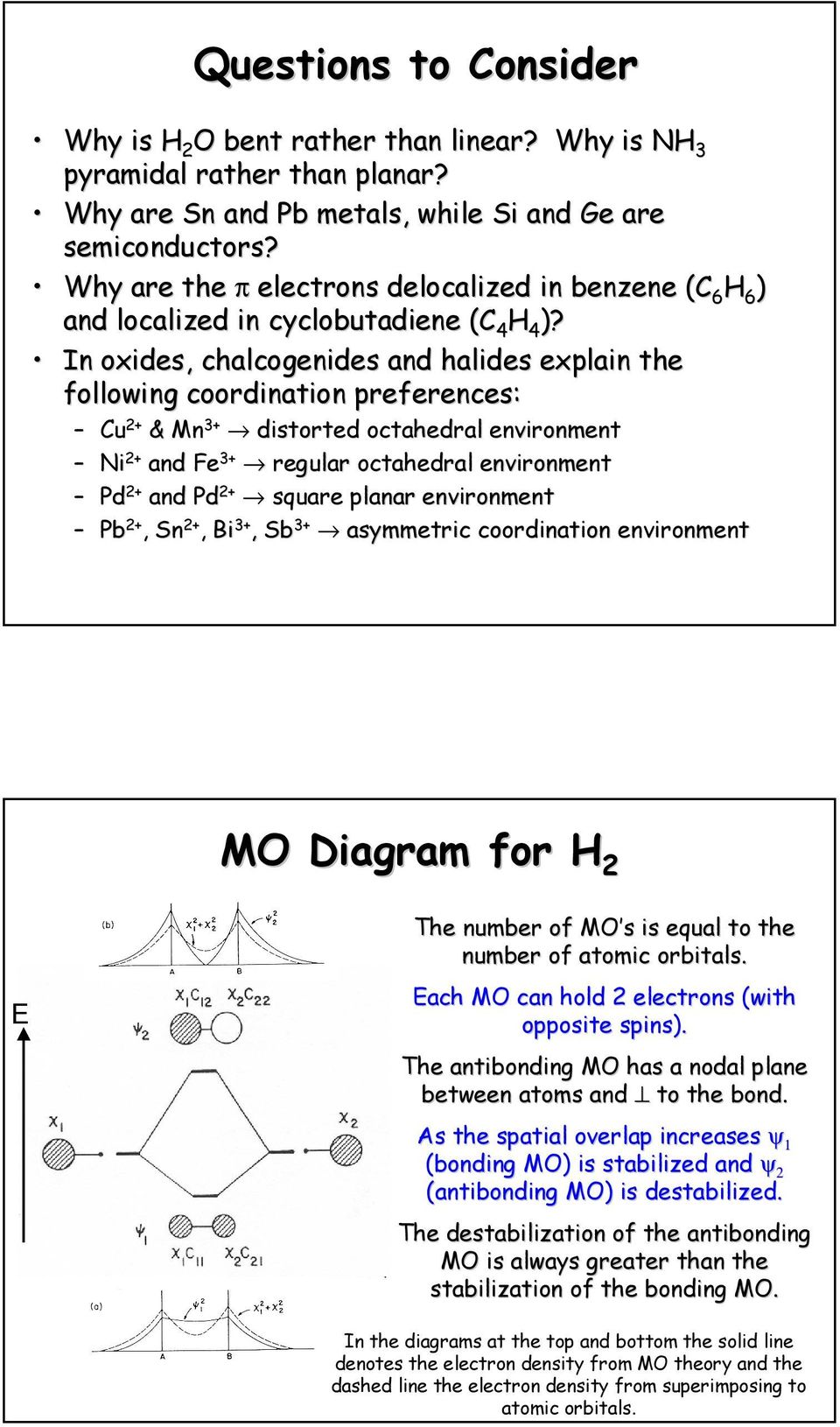
Rationalize why it is trigonal pyramidal by comparing the appropriate molecular orbital diagrams. 26 See the Walsh Diagram in ab for both confirmations,. -LUMO ... Draw the Lewis structure for N2. How does the molecular orbital bonding model correlate with the Lewis Model? Page 2. Chemistry Worksheet.
19. Refer to the MO Diagrams. Use molecular orbital theory to determine if the molecular are paramagnetic or diamagnetic. c. Li2 d. N2 a. F2 20. Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, determine bond order for each below. c. NO ...

Molecular orbital diagram practice worksheet
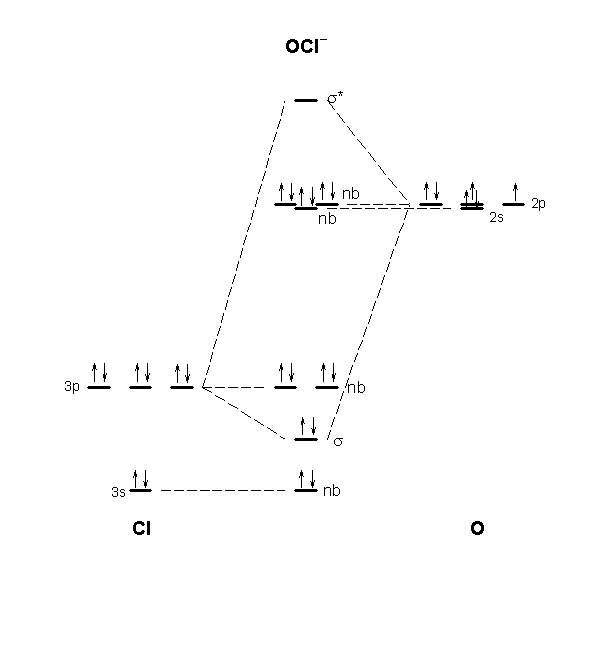
Write a complete electronic configuration and orbital diagram for zirconium, Zr. Molecular Orbital Theory Worksheet 1. Sketch the molecular orbital diagram of Hz-. Write the electron configuration of the ions in terms of its MOu0026#39;s. Worksheet 2 Notes Dr. Richard Nafshun - Welcome to the OSU ... The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated. diagram that the EHOMO-LUMO gap of a pi bond is larger than the EHOMO-LUMO in the conjugated pi system of the diene. Simple conjugation of two pi bonds, producing four molecular orbitals, (MOs). nodes are breaks in electron density (= higher energy orbital) LUMO = lowest unoccupied molecular orbital 1 = ( 1 + 2)
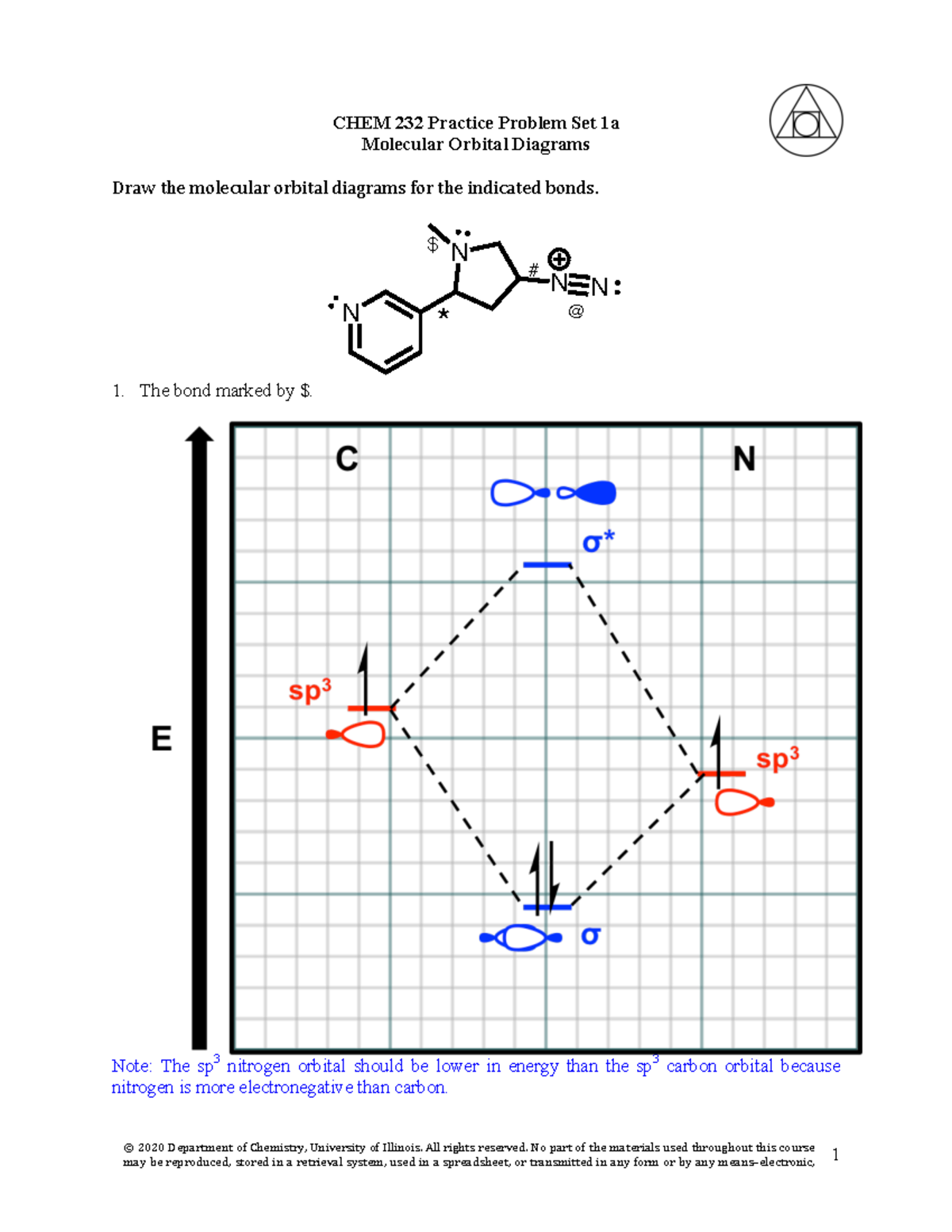
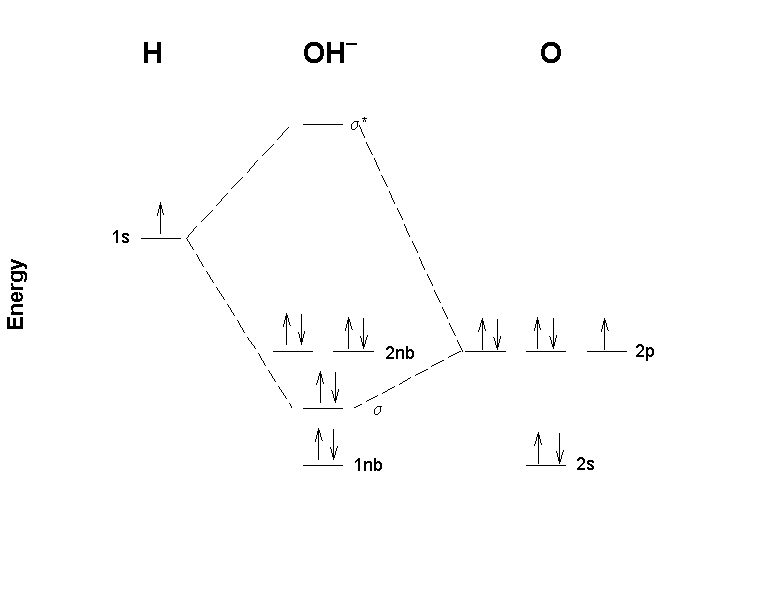
Molecular orbital diagram practice worksheet. Chapter 11 Answers Practice Examples 1a. There are three half-filled 2p orbitals on N, and one half-filled 5p orbital on I. Each half-filled 2p orbital from N will overlap with one half-filled 5p orbital of an I. Thus, there will be three N—I bonds. The I atoms will be oriented in the same direction as the three 2p orbitals of N: toward the x ,y, and z-directions of a Cartesian coordinate ... Molecular orbital Diagram Practice. molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each molecule has a set of molecular orbitals ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Answers to Practice Test Questions 3 . Molecular Orbital Theory: Heteronuclear Diatomic Molecules . 1. (a) 1The electron configuration for 𝐻𝐻 is 1𝑠𝑠, so 𝐻𝐻 has 1 valence electron. ... The MO diagram shows three pairs of nonbonding electrons in MOs localized on Cl. The two bonding theories therefore give the same picture of ...
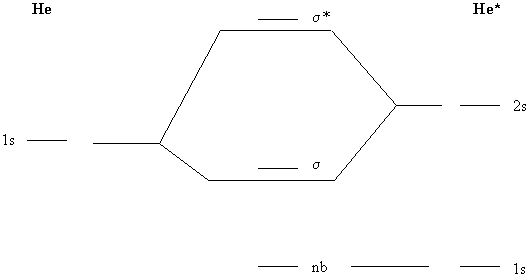
LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb Anti-bonding orbitals have a nodal plane between the nuclei. An asterisk (“*”) is added to the σ or π label to show this. The MO diagrams for homonuclear ... 2. Draw the four π-molecular orbitals for the molecule butadiene, drawn below. Label . the four orbitals as bonding, non-bonding or antibonding. Which of the four orbitals . are occupied? Which is the LUMO and which is the HOMO? 3. Draw the six π-molecular orbitals for benzene, label them as bonding non-bonding or . antibonding. Which are ... Displaying top 8 worksheets found for - Orbital. The number of molecular orbitals created by. Using arrows show how the following orbitals will fill with electrons. What is the ml values for the following types of orbitals. Worksheet 14 - Hybridization When atoms bond to form molecules they use molecular orbitals.
Print Molecular Orbital Theory: Tutorial and Diagrams Worksheet 1. What happens when both of the orbitals in a molecule are in phase, either both positive or both negative, and the electrons in ... CHEM 2000 Exercises and Practice Test Questions. Exercises are short focused sets of practice questions that can be printed and used as worksheets. Each Exercise focuses on a single concept or skill. You should complete Exercises immediately after the concept or skill is discussed in class to ensure that you fully understand it so that you do ... together to produce a sigma molecular orbital [σ = (1sa + 1sb)]. Since the electrons in this orbital are more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This ... MO Diagram: 2s. 2pz b. The energy level diagrams for CH2 and BeH2 feature the same orbital interactions. One difference is that the different number of ...
Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, ...
Chemistry orbital diagram worksheet molecular orbital diagram worksheet orbital diagram practice worksheet orbital diagram worksheet orbital diagram worksheet 1 orbital diagram worksheet 5 5 orbital diagram worksheet 5 5 answers orbital. And that actinium ac is the first element in the 5f block. Is 2s lectron is 4s on 2s a o o gurations or ome ...
6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
p. The energy diagram for this process is shown below. The hybridized orbitals are higher in energy than the s orbital, but lower in energy than the p orbitals, following Hundls rule. atomic orbitals sp hybridization hybridized orbitals Carbon has 4 valence electrons. Add these electrons to the atomic and molecular orbitals.
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Practice energy diagrams for molecular orbital theory. Calculate the number of bonding and antibonding electrons in simple molecules. Calculate bond order for simple molecules. Determine the magnetism of simple molecules.
The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
HÜCKEL MOLECULAR ORBITAL THEORY In general, the vast majority polyatomic molecules can be thought of as consisting of a collection of twoelectron bonds between pairs of atoms. So the qualitative picture of σ and πbonding and antibonding orbitals that we developed for a diatomic like CO can be carried over give a qualitative
Manual for daewoo 1550xl skid steer orbital diagram chem worksheet answers gehl axles as orbital diagram chem worksheet 5 5 answers coloring pages math skills for kids games4kids value of numbers with. Orbital practice worksheet. Nsm 12c pal worksheet molecular orbital theory name: define the following sigma orbital:. Electron orbital ...
Molecular Orbital Diagram Practice. February 15, 2015 . By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules.
Day 8 Molecular Orbital Theory Part 3 1 Inorganic Chemistry with Doc M. Day 8. Molecular Orbitals: Symmetry adapted linear combinations, SALCs Topics: 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals 2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4.
Lesson Worksheet: Molecular Orbital Theory. In this worksheet, we will practice describing the shapes, energies and electron occupancies of bonding, nonbonding, and antibonding molecular orbitals. An sp 2 hybridized atom in a molecule forms 𝜎 bonds to three other atoms of the same element. There are no other bonds in the molecule.
In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. ... Practice energy diagrams for molecular orbital theory. Calculate the number of bonding and ...
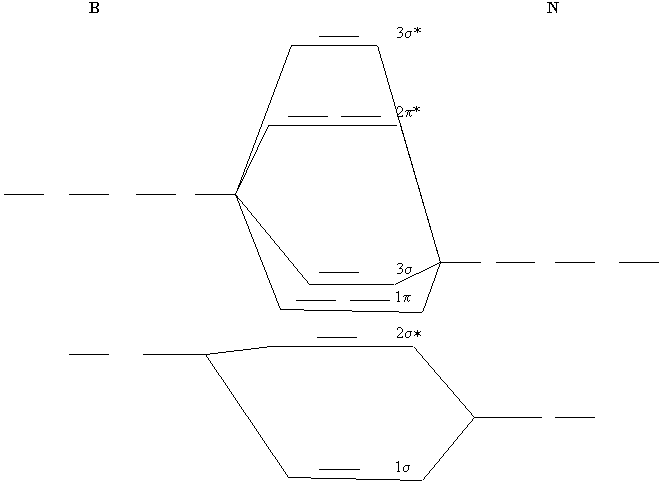
Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing.
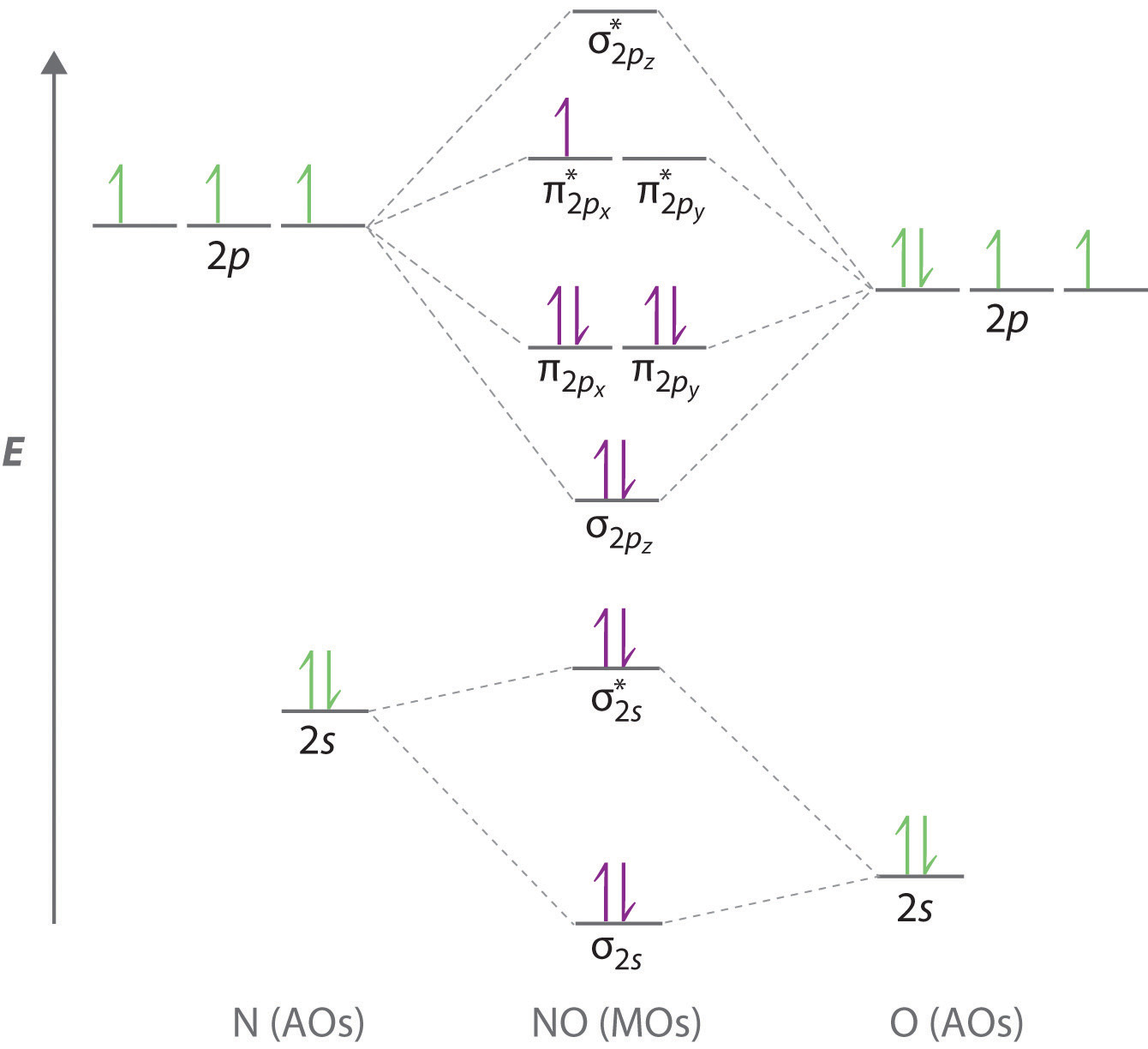
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
However, the diagram will still yield the correct bond order and magnetic behavior for these molecules. 1. Refer to the Molecular Orbital diagram above.
Showing top 8 worksheets in the category orbital diagrams. These problems are for practice in drawing your molecular orbital diagrams molecular electron configurations and determining bond order. Draw the molecular orbital diagram for n2 ion and calculate the bond order. Western connecticut state university molecular orbital theory.

Molecular Orbital Theory Chemistry Libretexts In 2021 Chemistry Textbook Geometry Worksheets Electron Configuration
diagram that the EHOMO-LUMO gap of a pi bond is larger than the EHOMO-LUMO in the conjugated pi system of the diene. Simple conjugation of two pi bonds, producing four molecular orbitals, (MOs). nodes are breaks in electron density (= higher energy orbital) LUMO = lowest unoccupied molecular orbital 1 = ( 1 + 2)
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
Write a complete electronic configuration and orbital diagram for zirconium, Zr. Molecular Orbital Theory Worksheet 1. Sketch the molecular orbital diagram of Hz-. Write the electron configuration of the ions in terms of its MOu0026#39;s. Worksheet 2 Notes Dr. Richard Nafshun - Welcome to the OSU ...

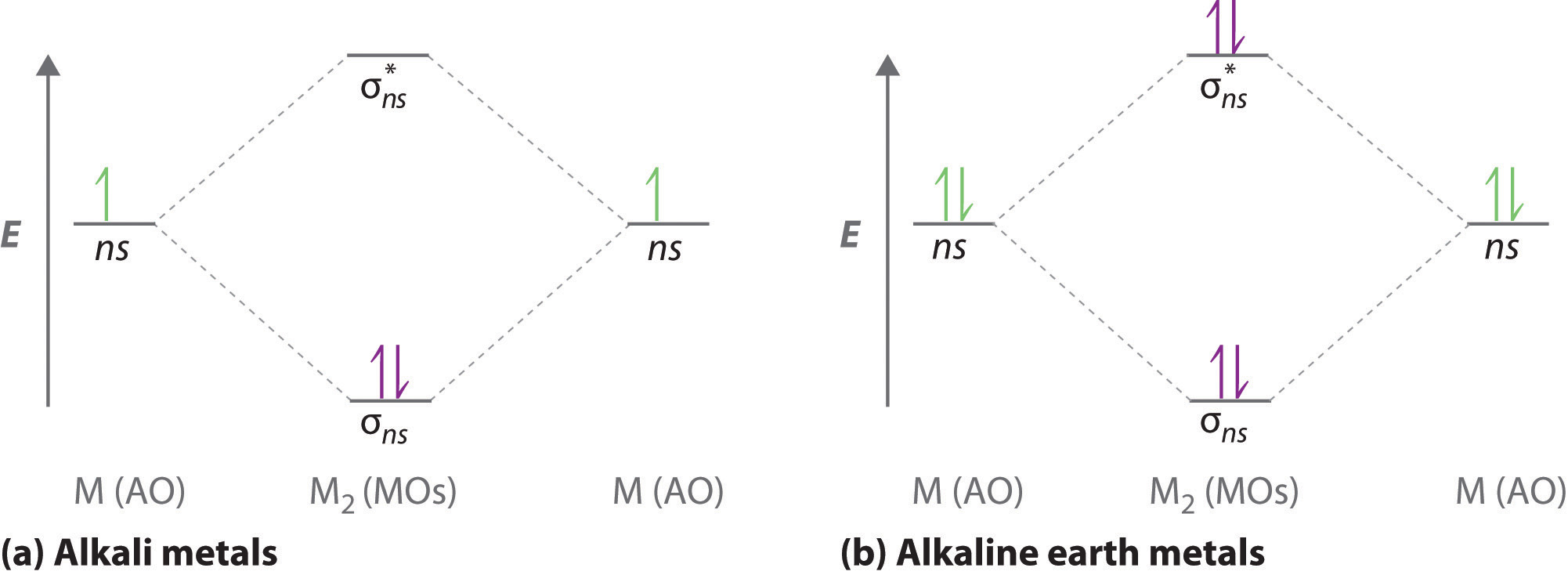

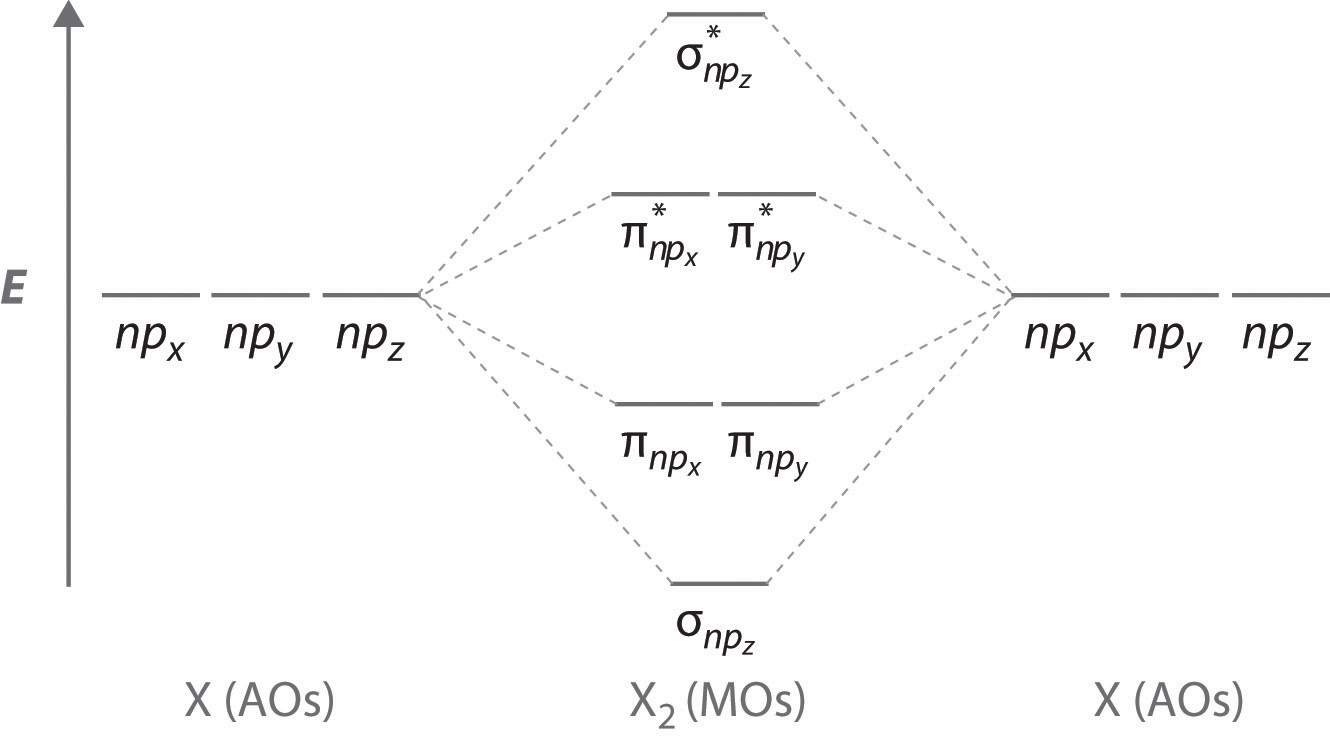
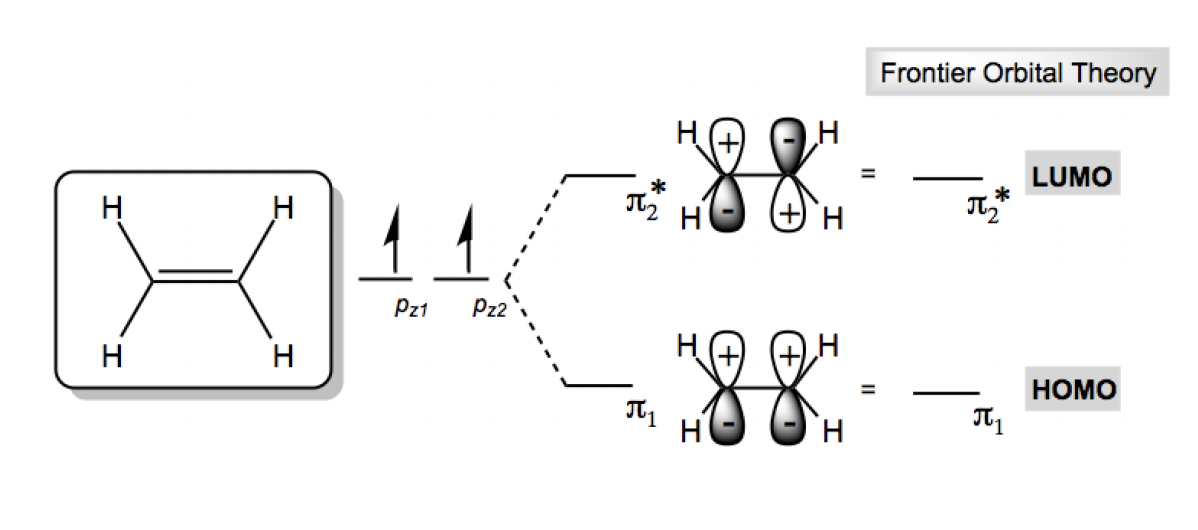




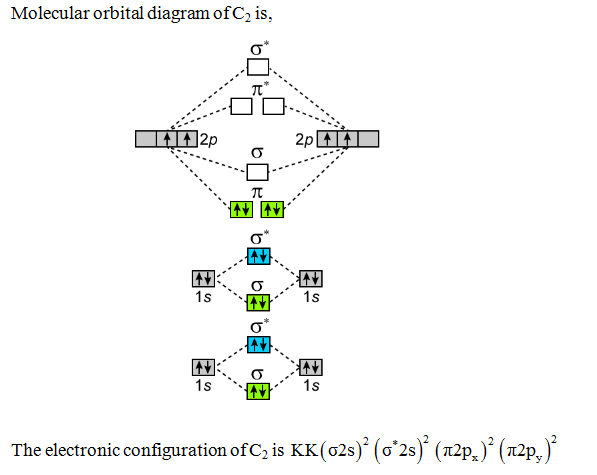





0 Response to "37 molecular orbital diagram practice worksheet"
Post a Comment