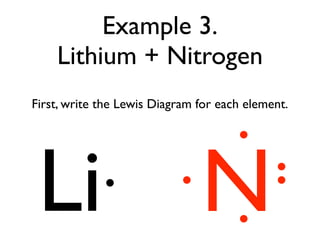
40 lewis dot diagram of nitrogen
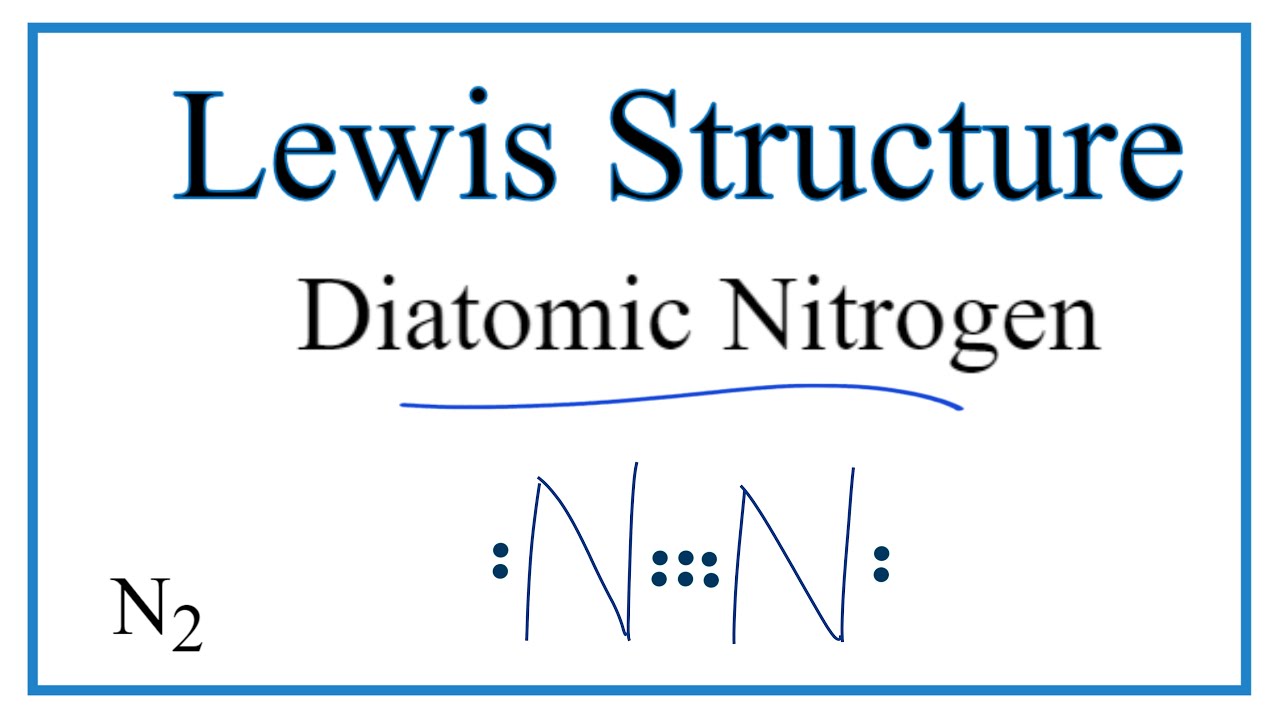
Nitrogen (N2) Molecule Lewis Structure Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen trifluoride (NF3) lewis dot structure, molecular ... So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX3N1 So, according to the VSEPR chart, if the molecule has the formula of AX3N1, it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral.
Lewis Structure of N2 (Nitrogen Gas) - YouTube How to Draw the Lewis Structure of N2 - with explanation!Check me out:

Lewis dot diagram of nitrogen
N2 Lewis Structure: Full Guide (2022 Updated) Jan 31, 2022 · Steps In Drawing the N2 Lewis Structure. To create a Lewis structure, determine first the number of valence electrons in each atom. Nitrogen has a total of ten valence electrons—five electrons on its outermost valence shell. After determining the total number of valence electrons., connect the atoms between electron pairs. How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ... N2 Lewis Structure - Easy Hard Science - Learn With Dr ... Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence ...
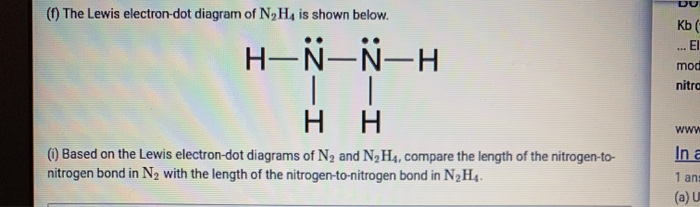
Lewis dot diagram of nitrogen. NO2 (Nitrogen Dioxide) Lewis Dot Structure | Science Trends NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3. What is Lewis dot diagram of nitrogen gas? - Answers The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . . How to Draw the Lewis Dot Structure for Diatomic Nitrogen ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Diatomic Nitrogen).For the N2 structure use the periodic table to find the total number... Draw the Lewis structure for N2. Nitrogen is an unreactive ... The lewis structure of n2h2 shows c. each nitrogen has one nonbinding electron pair Lewis structures are diagrams that show the bonds between the atoms of a molecule and the lone pair of electrons that might exist in a molecule. Further Explanation. Lewis structures can be drawn for each covalently bonded molecule, as well as coordination ...
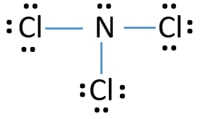
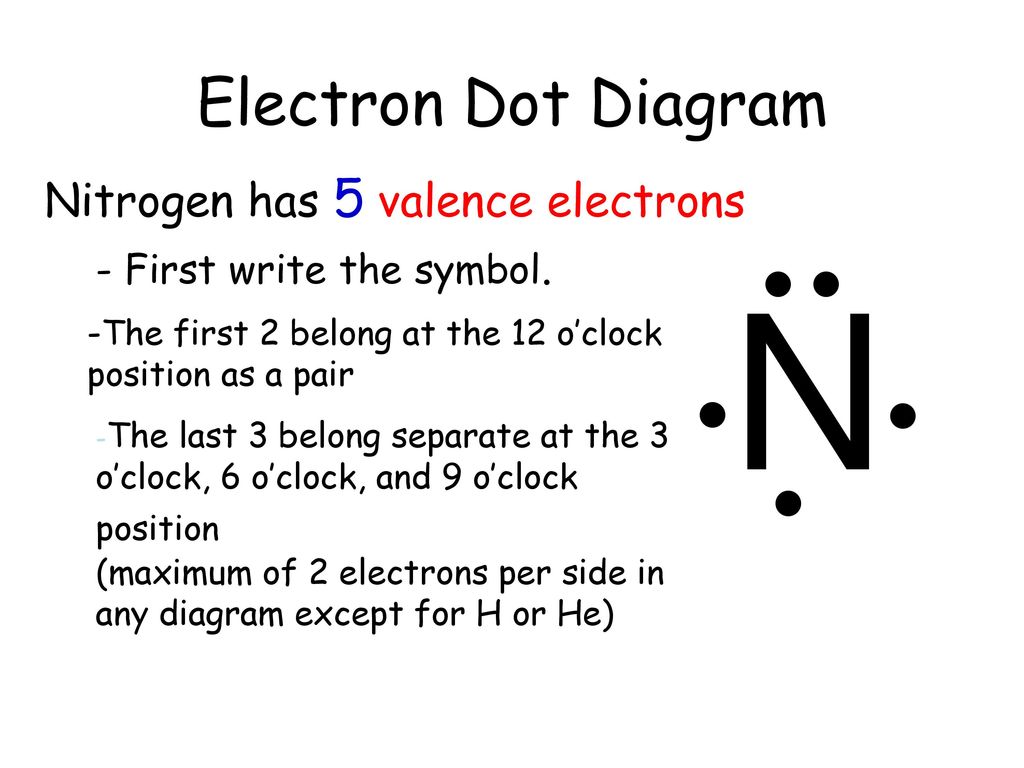
Lewis Dot Structure for Nitrogen Atom (N) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ... NF3 (Nitrogen trifluoride) Lewis Structure - Steps of Drawing NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.Each fluorine atom has three lone pairs. Steps of drawing lewis structure of NF 3. You have to follow few steps to draw the lewis structure of NF 3.Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps ... Lewis Dot Diagram For N2 - schematron.org The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. Draw and explain the Lewis dot structure of nitrogen ... Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn about Lewis dots and understand single bonds, double ...
NI3 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 structure use the periodic table to find the total num... Lewis Dot Diagram Of Nitrogen - schemacheck.com The Lewis dot structure of Nitrogen is shown below. This shows 5 dots around the symbol of nitrogen. These five dots represent the five electrons that. Using Lewis dot structures and the octet rule, we can predict and represent For diatomic nitrogen, the Lewis-dot structure correctly predicts that there. What is the Lewis dot structure for nitrogen gas ... Oct 19, 2020 · What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. What is the Lewis dot structure for nitrogen ... What is the dot structure of NO2? The NO2 Lewis structure has a total of 17 valence electrons. It’s not common to have an odd number of valence electrons in a Lewis structure. Because of this we’ll try to get as close to an octet as we can on the central Nitrogen (N) atom.
How to Draw the Lewis Dot Structure for Ba3N2: Barium ... A step-by-step explanation of how to draw the Ba3N2 Lewis Dot Structure.For Ba3N2 we have an ionic compound and we need to take that into account when we dra...
NO2 (Nitrogen Dioxide) Lewis Dot Structure NO2 (Nitrogen Dioxide) Lewis Dot Structure. 27/02/2022. Global Tech News Daily. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom.
Kami Export - Lewis-dot-diagram-worksheet.pdf - Name Date ... View Kami Export - Lewis-dot-diagram-worksheet.pdf from SCIENCE 10 at Ramsay High Sch. Name Date Block Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons.
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
N2 Lewis Structure - Easy Hard Science - Learn With Dr ... Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence ...
How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ...
N2 Lewis Structure: Full Guide (2022 Updated) Jan 31, 2022 · Steps In Drawing the N2 Lewis Structure. To create a Lewis structure, determine first the number of valence electrons in each atom. Nitrogen has a total of ten valence electrons—five electrons on its outermost valence shell. After determining the total number of valence electrons., connect the atoms between electron pairs.

![Expert Answer] Draw the electron dot structure of nitrogen ...](https://hi-static.z-dn.net/files/d97/49b94f9b38ffa7a775377327493f1d68.png)



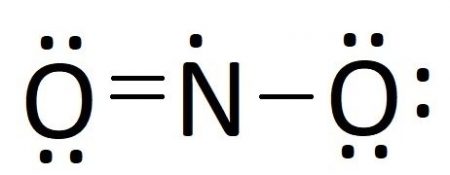
![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1648865_1784763_ans_65460fbc5eda4a3b8021b75bbc2803a5.png)







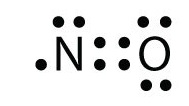




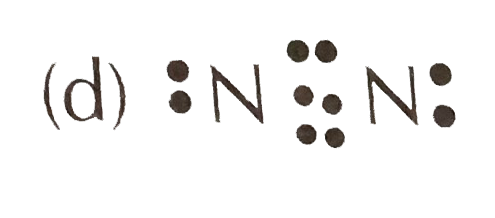







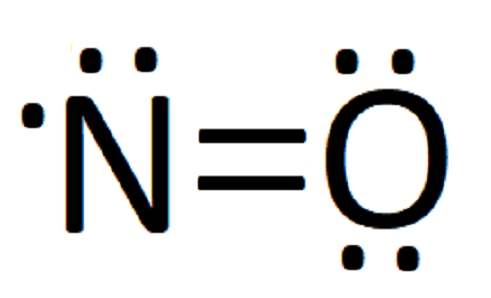

0 Response to "40 lewis dot diagram of nitrogen"
Post a Comment